HYPOTHESIS IN THROMBOSIS!
THE NOTION THAT INFLAMMATORY BOWEL DISEASE (IBD) CAN BE EXACERBATED BY
NON STEROIDAL ANTI-INFLAMMATORY DRUGS (NSAID), opens the door to speculation that in a certain set of patients, NSAID can precipitate a catastrophic event including stroke and heart attack or sudden death syndrome.
IBD seems to include a thrombostatic state full of risk for thrombosis formation therefore the hypothesis does not seems far fetched. In patients with IBD, the level of IL23 seems to be elevated. And the disease appears to be linked to this. IS THERE JUSTIFICATION TO RECOMMEND THAT BEFORE PUTTING PATIENT ON aSPIRIN, THAT THE LEVEL OF INTERLEUKIN-23 BE TESTED?
IT HAS BEEN ALSO SUGGESTED THAT THE EXACERBATION OF IBD IS DUE TO THE SUPPRESSION OF A COUNTER-BALANCING CYTOKINE, MAY BE INTERLEUKIN-4. SHOULD WE BE MONITORING LEVEL OF INTERLEUKIN-4 TO PREDICT THE RISK OF FURTHER THROMBOSIS IN CORONARY ARTERY DISEASE? IS HIGH INTERLEUKIN-4 PROTECTIVE OF FURTHER THROMBOSIS? WHAT CAUSES EXTENSION OF THROMBOSIS?
A blog about research, awareness, prevention, treatment and survivorship of Breast Cancer and all cancers, including targeted scientific research and a grassroots approach to increase screening for cancer, especially in the low income and under-insured population of El Paso, Texas, with a view to expand this new health care model to many other 'minority' populations across the United States and beyond
Tuesday, June 25, 2013
Sunday, June 23, 2013
PARADIGM SHIFT NEEDED IN PREVENTION MEDICINE!
IF ONE CAN DIE BECAUSE A DYSFUNCTION IN THE NOTCH OR THE WNT PATHWAYS? WHY MONITOR CHOLESTEROL!
AN NHI RFA ASKED:" In particular, “sudden death” among dialysis patients, even in the pediatric age range, is largely unexplained and needs further study.". Well we monitor in Chronic renal failure K,Na,Ca,BUN,Creatinine,GFR,cholesterol,PTH...how come we still have these sudden death. Nobody checked the status of amplification on the Wnt, the NOTCH, PI3K,AKT, NITRIC OXIDE, which we know to be affected in this disease. I tell you right now this will be medicine of the future!
Case in point: The trade off Hypothesis
----------------------------------------
"When Chronic Kidney disease impairs the kidney's ability to excrete phosphate, Phosphate accumulate in the intracellular and extracellular fluid and lead to the physicochemical formation of Calcium-phospate complexes that reduce the level of ionized calcium". Low serum calcium will trigger release of PTH which suppress the reabsorption of Phosphate by the proximal tubule of the kidney and increase renal excretion of phosphate but as the process continue, the level of Phosphate drops and the relative increase of Calcium suppresses the PTH but the new steady state is achieved at a higher PTH threshold. The recognition that a higher PTH is needed tells us that other receptor susceptible to PTH are now Hyperstimulated. At Bone level, the bone continue to be affected and RENAL BONE DISEASE WILL PROGRESS EVEN AS CALCIUM AND PHOSPHATE ARE CORRECTED IN THE SERUM!
QUESTION FOR YOU, WHAT NEEDS TO BE MONITORED, CALCIUM OR PHOSPHATE Vs THE PTH GENE?
OVER EXPRESSION OF A GENE WILL IMPACT OTHER GENES AND UNTIL THAT OVEREXPRESSION IS CORRECTED WE HAVE NOT ACCOMPLISHED MUCH PREVENTION!
UNTIL WE START MONITORING GENE EXPRESSION, AMPLIFICATION OR SUPPRESSION, WE ARE NOWHERE IN PREVENTIVE MEDICINE FOR STROKE, CORONARY ARTERY DISEASE OR DIABETES MELITUS FOR THAT MATTER!
IF ONE CAN DIE BECAUSE A DYSFUNCTION IN THE NOTCH OR THE WNT PATHWAYS? WHY MONITOR CHOLESTEROL!
AN NHI RFA ASKED:" In particular, “sudden death” among dialysis patients, even in the pediatric age range, is largely unexplained and needs further study.". Well we monitor in Chronic renal failure K,Na,Ca,BUN,Creatinine,GFR,cholesterol,PTH...how come we still have these sudden death. Nobody checked the status of amplification on the Wnt, the NOTCH, PI3K,AKT, NITRIC OXIDE, which we know to be affected in this disease. I tell you right now this will be medicine of the future!
Case in point: The trade off Hypothesis
----------------------------------------
"When Chronic Kidney disease impairs the kidney's ability to excrete phosphate, Phosphate accumulate in the intracellular and extracellular fluid and lead to the physicochemical formation of Calcium-phospate complexes that reduce the level of ionized calcium". Low serum calcium will trigger release of PTH which suppress the reabsorption of Phosphate by the proximal tubule of the kidney and increase renal excretion of phosphate but as the process continue, the level of Phosphate drops and the relative increase of Calcium suppresses the PTH but the new steady state is achieved at a higher PTH threshold. The recognition that a higher PTH is needed tells us that other receptor susceptible to PTH are now Hyperstimulated. At Bone level, the bone continue to be affected and RENAL BONE DISEASE WILL PROGRESS EVEN AS CALCIUM AND PHOSPHATE ARE CORRECTED IN THE SERUM!
QUESTION FOR YOU, WHAT NEEDS TO BE MONITORED, CALCIUM OR PHOSPHATE Vs THE PTH GENE?
OVER EXPRESSION OF A GENE WILL IMPACT OTHER GENES AND UNTIL THAT OVEREXPRESSION IS CORRECTED WE HAVE NOT ACCOMPLISHED MUCH PREVENTION!
UNTIL WE START MONITORING GENE EXPRESSION, AMPLIFICATION OR SUPPRESSION, WE ARE NOWHERE IN PREVENTIVE MEDICINE FOR STROKE, CORONARY ARTERY DISEASE OR DIABETES MELITUS FOR THAT MATTER!
STRONG GENES ARE ASSOCIATED WITH MALFORMATIONS
================================================
Times and times again in the life of this blog, we continue to stress that the power of a gene is revealed when its deficiency, lack of function or mutation leads to a Malformation. Another example of this is the Androgen or steroid related gene. Women with Inflammatory bowel disease who are pregnant and given steroids have either early/premature rupture of membranes and/or their infants have a higher rates of cleft lip!
TISSUES SHARE SAME GENES !
=================================
Whenever you target a gene with target therapy treatment, always keep in mind that genes are the same in all cells! Patterns of gene silencing in various tissues determine the expression of same genes in various tissues. And do remember that some mechanisms of silencing are irreversible and some are reversible. It is interesting that in irreversible cases not only the cell knows that the gene is silenced it keeps memory of this, meaning de-methylation of some genes in a particular tissue has no effect into new expression of that gene, as if not only the cell Methylate a gene to silence it, it also remove proteins that helps its expression once demethylated!
It is striking that expressed genes finding themselves targeted by therapy in the white blood cells, are also active in the brain and liver, leading to liver failure and and various Central Nervous system (CNS) side effects ranging from coma to LEUCO-ENCEPHALOPATHY! IT IS UNCLEAR WHETHER STUDYING THE LEVER OF AMPLIFICATION OF TLE4, OR STATUS OF THE GROUCHO FAMILY MEMBER COULD HELP PREDICT WHO WILL MORE LIKELY DEVELOP CNS SIDE EFFCT!
================================================
Times and times again in the life of this blog, we continue to stress that the power of a gene is revealed when its deficiency, lack of function or mutation leads to a Malformation. Another example of this is the Androgen or steroid related gene. Women with Inflammatory bowel disease who are pregnant and given steroids have either early/premature rupture of membranes and/or their infants have a higher rates of cleft lip!
TISSUES SHARE SAME GENES !
=================================
Whenever you target a gene with target therapy treatment, always keep in mind that genes are the same in all cells! Patterns of gene silencing in various tissues determine the expression of same genes in various tissues. And do remember that some mechanisms of silencing are irreversible and some are reversible. It is interesting that in irreversible cases not only the cell knows that the gene is silenced it keeps memory of this, meaning de-methylation of some genes in a particular tissue has no effect into new expression of that gene, as if not only the cell Methylate a gene to silence it, it also remove proteins that helps its expression once demethylated!
It is striking that expressed genes finding themselves targeted by therapy in the white blood cells, are also active in the brain and liver, leading to liver failure and and various Central Nervous system (CNS) side effects ranging from coma to LEUCO-ENCEPHALOPATHY! IT IS UNCLEAR WHETHER STUDYING THE LEVER OF AMPLIFICATION OF TLE4, OR STATUS OF THE GROUCHO FAMILY MEMBER COULD HELP PREDICT WHO WILL MORE LIKELY DEVELOP CNS SIDE EFFCT!
Saturday, June 22, 2013
KEEPING YOU IN THE LOOP!
The
Indiana State Department of Health, in collaboration with the Marion
County Public Health Department, Indiana Hospital Association,
Association for Professionals
in Infection Control and Epidemiology (Indiana Chapter), IU Health, and
The New Wishard Eskenazi Health Hospital, has convened the Indiana
Antibiotic Resistance Advisory Committee. The Committee is administering
this survey to health care providers and hospital
administrators to assess awareness of treatment, prevention, and
antibiotic stewardship for multi-drug resistant organisms (MDRO),
including
Clostridium difficile, methicillin-resistant Staphylococcus aureus,
Mycobacterium tuberculosis, and most recently, carbapenem-resistant Enterobacteriaceae (CRE).
Enterobacteriaceae, a family of bacteria that normally live in the human gut, includes organisms such as Klebsiella species and Escherichia coli. CRE have developed resistance to almost all antibiotics, including broad-spectrum agents called carbapenems. Within the past 10 years, the incidence of CRE has been increasing across the United States. CRE infections are difficult to treat and associated with a high attributable mortality.
The survey, accessible through the SurveyMonkey link below, will take about 15-20 minutes to complete. The survey includes a brief demographic section, a section specific to CRE, and a section with questions on MDRO in general. Your input is very important, as survey data will be shared in aggregate form and allow the committee to establish priorities and develop educational materials.
Please complete this survey by the close of business on Thursday, July 11, 2013. Thank you very much for your participation!
Enterobacteriaceae, a family of bacteria that normally live in the human gut, includes organisms such as Klebsiella species and Escherichia coli. CRE have developed resistance to almost all antibiotics, including broad-spectrum agents called carbapenems. Within the past 10 years, the incidence of CRE has been increasing across the United States. CRE infections are difficult to treat and associated with a high attributable mortality.
The survey, accessible through the SurveyMonkey link below, will take about 15-20 minutes to complete. The survey includes a brief demographic section, a section specific to CRE, and a section with questions on MDRO in general. Your input is very important, as survey data will be shared in aggregate form and allow the committee to establish priorities and develop educational materials.
Please complete this survey by the close of business on Thursday, July 11, 2013. Thank you very much for your participation!
William C. VanNess II, MD
State Health Commissioner
A CASE IN POINT WALKED IN, GENETIC AT WORK!
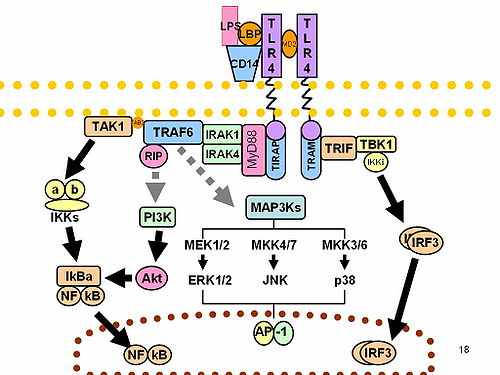
==========================================RECEPTOR FAILURE LEADS TO THE CROHN'S SYNDROME.
Last night at 1:am I received a call from the Emergency Room at a local hospital, a 29 year old mother of 4, under some stress had been taking Advil 6 tablets a day for Headache for 3-4 weeks, she now presented to the emergency room with 5 days of diarrhea and fever, with Hypokalemia (K=3.0) and severe Right lower Quadran of the Abdomen. An appendicitis was suspected and a CT of the abdomen and pelvis was obtained suggesting changes consistent with Appendicitis but most significant is a thick swollen initial colon (CECUM) with some surrounding "fluid" and minimal surrounding Adenopathies. A surgical consultation has been obtained, the surgeon however is advising input first from a Colonoscopy. We are waiting for the Gastroenterologist input as we speak! The patient is NPO and Cipro-and IV Flagyl is being given, and further laboratory results are awaited!
If this case turns out to be an inflammatory bowel disease, It will be a classic reportable case!
Indeed "Non steroidal anti-inflammatory drugs (NSAID-in this case Advil taken for Headache) appear to be associated with exacerbations of disease". (GARY LICHTEINSTEIN)
First thing to remember as we discussed failure of receptors as a cause of Triple negative Breast cancers where defect in Heparan sulfate the Glycocalyx covering receptor on breast tissue was a cause. Here in the terminal Ileum and early Cecum, it is the Muramyl Dipeptide, a Peptidoglycan component of gram positive bacteria which fails to interact with a Mutated NOD2 gene product. And these failure of stimulation at cellular levels are a potential irritant to the stress related c-JUN, and to production of Heat stroke proteins (most likely HSP90) and secondary stimulation of the NF-kB which leads to Cytokines. In Inflammatory Bowel diseases, The cytokyne of choice is IL23, its opposing cytokine (may be IL4) knocked down by the NSAID, leads to a significant exacerbation of disease!
There is susceptility conferred by class II antigen DR molecule. And of interest is the found Hypokalemia which result from Cation related ion-channel disturbance linked to the OCTN1 GENE. "OCTN1,LOCATED ON 5q31, codes for an ion channel". The DLG5 is involved. It "codes for a scaffolding protein that is important for maintaining epithelial integrity in various organs".
WE WILL INFORM YOU WHEN ALL IS SAID AND DONE IN THIS INTERESTING CASE!
==========================================RECEPTOR FAILURE LEADS TO THE CROHN'S SYNDROME.
Last night at 1:am I received a call from the Emergency Room at a local hospital, a 29 year old mother of 4, under some stress had been taking Advil 6 tablets a day for Headache for 3-4 weeks, she now presented to the emergency room with 5 days of diarrhea and fever, with Hypokalemia (K=3.0) and severe Right lower Quadran of the Abdomen. An appendicitis was suspected and a CT of the abdomen and pelvis was obtained suggesting changes consistent with Appendicitis but most significant is a thick swollen initial colon (CECUM) with some surrounding "fluid" and minimal surrounding Adenopathies. A surgical consultation has been obtained, the surgeon however is advising input first from a Colonoscopy. We are waiting for the Gastroenterologist input as we speak! The patient is NPO and Cipro-and IV Flagyl is being given, and further laboratory results are awaited!
If this case turns out to be an inflammatory bowel disease, It will be a classic reportable case!
Indeed "Non steroidal anti-inflammatory drugs (NSAID-in this case Advil taken for Headache) appear to be associated with exacerbations of disease". (GARY LICHTEINSTEIN)
First thing to remember as we discussed failure of receptors as a cause of Triple negative Breast cancers where defect in Heparan sulfate the Glycocalyx covering receptor on breast tissue was a cause. Here in the terminal Ileum and early Cecum, it is the Muramyl Dipeptide, a Peptidoglycan component of gram positive bacteria which fails to interact with a Mutated NOD2 gene product. And these failure of stimulation at cellular levels are a potential irritant to the stress related c-JUN, and to production of Heat stroke proteins (most likely HSP90) and secondary stimulation of the NF-kB which leads to Cytokines. In Inflammatory Bowel diseases, The cytokyne of choice is IL23, its opposing cytokine (may be IL4) knocked down by the NSAID, leads to a significant exacerbation of disease!
There is susceptility conferred by class II antigen DR molecule. And of interest is the found Hypokalemia which result from Cation related ion-channel disturbance linked to the OCTN1 GENE. "OCTN1,LOCATED ON 5q31, codes for an ion channel". The DLG5 is involved. It "codes for a scaffolding protein that is important for maintaining epithelial integrity in various organs".
WE WILL INFORM YOU WHEN ALL IS SAID AND DONE IN THIS INTERESTING CASE!
Friday, June 21, 2013
YOU BE THE JUDGE OF THIS CRYPTIC COMMUNICATION:
![The White House, Washington]()
Hi, all!
We wanted to share some really good news for consumers about health care costs so that you can also help to get out the word.
First, health insurance premium rebates, which average nearly $100 per family, are on their way to 8.5 million Americans. That is because the Affordable Care Act now holds health insurance companies accountable to consumers and ensures that American families receive value for their premium dollars. If an insurance company spends less than 80 percent (85 percent in the large group market) of your premium on medical care and efforts to improve the quality of care and instead spends it on overhead and corporate salaries, they must rebate the portion of premium that exceeded this limit. Thanks to the law, millions of Americans will get savings from their insurers.
The law is protecting consumers from rate hikes that don’t go to care. It, along with other provision of the Affordable Care Act, has already helped consumers save a total of $3.9 billion in 2012, and $5 billion counting the 2011 rebates.
Second, Americans know health care prices for decades were skyrocketing, but the health law is changing that. This week, we learned that the official measure of health care prices, the medical price index, fell in May. The average for the last year was the lowest it has been in over four decades -- a sign that health care is getting more affordable.
And third, a new report demonstrates this good news will continue. A new PriceWaterhouseCooper report projects that medical cost growth will be lower in 2014 than in 2013. As they say: “defying historical patterns.” The report points to how the ACA, though its policies to promote value, reduce unnecessary care, and cut waste from the system, is driving down the cost of health care for all of us.
Meanwhile, we received a number of questions in response to our last email. Here are a couple answers:
If the pre-existing limitations are no longer allowed can an insurance company still decline to cover an applicant?
No. No new applicants may be denied coverage due to a pre-existing condition starting in 2014, and permanently thereafter. Insurance companies will also be prohibited from charging more or carving out health benefits due to a pre-existing. Charging women more will become a thing of the past. And you won’t pay more based on the type of work you do or your family’s health history.
How does the ACA help people on Medicare?
People with Medicare now have free checkups and preventive services – you don’t have to be sick to see your doctor to stay healthy. They have a new discount on their prescription medicine in the so-called “donut hole” that’s already saved over six million seniors more than $700 each. The increases in their monthly Medicare premiums, like those for private insurance, have slowed down – with the Medicare Trustees projecting that they may actually drop in 2014. And, the part of the law that prevents insurance companies from spending premium dollars on overhead not care will begin in the Medicare Advantage next year, thanks to the Affordable Care Act.
We’ll tackle more of your questions in future emails, so be sure to let us know what’s on your mind.
Thanks!
Tara
Tara McGuinness
Senior Communications Advisor
The White House
=======================================================
CRBCM DOES NOT ENDORSE ANY POLITICAL SIDE!
THIS COMMUNICATION IS PUBLISHED FOR YOUR "NEED TO KNOW" !
Hi, all!
We wanted to share some really good news for consumers about health care costs so that you can also help to get out the word.
First, health insurance premium rebates, which average nearly $100 per family, are on their way to 8.5 million Americans. That is because the Affordable Care Act now holds health insurance companies accountable to consumers and ensures that American families receive value for their premium dollars. If an insurance company spends less than 80 percent (85 percent in the large group market) of your premium on medical care and efforts to improve the quality of care and instead spends it on overhead and corporate salaries, they must rebate the portion of premium that exceeded this limit. Thanks to the law, millions of Americans will get savings from their insurers.
The law is protecting consumers from rate hikes that don’t go to care. It, along with other provision of the Affordable Care Act, has already helped consumers save a total of $3.9 billion in 2012, and $5 billion counting the 2011 rebates.
Second, Americans know health care prices for decades were skyrocketing, but the health law is changing that. This week, we learned that the official measure of health care prices, the medical price index, fell in May. The average for the last year was the lowest it has been in over four decades -- a sign that health care is getting more affordable.
And third, a new report demonstrates this good news will continue. A new PriceWaterhouseCooper report projects that medical cost growth will be lower in 2014 than in 2013. As they say: “defying historical patterns.” The report points to how the ACA, though its policies to promote value, reduce unnecessary care, and cut waste from the system, is driving down the cost of health care for all of us.
Meanwhile, we received a number of questions in response to our last email. Here are a couple answers:
If the pre-existing limitations are no longer allowed can an insurance company still decline to cover an applicant?
No. No new applicants may be denied coverage due to a pre-existing condition starting in 2014, and permanently thereafter. Insurance companies will also be prohibited from charging more or carving out health benefits due to a pre-existing. Charging women more will become a thing of the past. And you won’t pay more based on the type of work you do or your family’s health history.
How does the ACA help people on Medicare?
People with Medicare now have free checkups and preventive services – you don’t have to be sick to see your doctor to stay healthy. They have a new discount on their prescription medicine in the so-called “donut hole” that’s already saved over six million seniors more than $700 each. The increases in their monthly Medicare premiums, like those for private insurance, have slowed down – with the Medicare Trustees projecting that they may actually drop in 2014. And, the part of the law that prevents insurance companies from spending premium dollars on overhead not care will begin in the Medicare Advantage next year, thanks to the Affordable Care Act.
We’ll tackle more of your questions in future emails, so be sure to let us know what’s on your mind.
Thanks!
Tara
Tara McGuinness
Senior Communications Advisor
The White House
=======================================================
CRBCM DOES NOT ENDORSE ANY POLITICAL SIDE!
THIS COMMUNICATION IS PUBLISHED FOR YOUR "NEED TO KNOW" !
GENETIC ISSUES IN LYMPHOPROLIFERATIVE DISORDERS, ASPECTS OF GENETIC BASED PATHOPHYSIOLOGY IN THIS DISEASE
Now it is established that lymphoma are due to a critical event causing Heavy chain IgH to be transposed into proximity with a promoter of PAX5 gene leading to 2 events
1. stimulation of TLE4 that force differentiation to lymphocyte proliferation. TLE4 is a tranduction enhancer which tell the cell no Brain differentiation by a negative regulation of the Groucho family members! but it does force proliferation of lymphoblasts forward.
2.to insure survival of lymphoblasts, it consumes or de-repress the Death protein 6 leaving FAS intact and therefore NO APOPTOSIS, and favoring an intact Kinechore plate to preserve speedy cell division by preserving the Centromere protein C. Decrease of the Death receptor has a significant 3rd effect on a series of TNF receptors and ligand which ultimately through ETS1 and TTRAP end up affecting CD40 leading to weakening of the cell presentation of Antigen which is the underlying immunodeficeinec in lymphoma (similar to hyper IgM Syndrome)
This is the underlying pathophysiology of Lymphoma as it looks today.
OF NOTE CD40 IS ALSO INVOLVED IN ALZHEIMER!
JUST REMEMBER THE CD40 THRU TRAF GENES INTERACTS WITH CD30 TO ACTIVATE THE NF-kB AND CYCLINS!
CRITICAL TARGET IN THIS PATHWAYS
1.PROTEIN QD
2,KINETOCHORE, CENTROMERE PROTEIN C
3.FAS
4.DEATH PROTEIN 6
5.TTRAP
6.CD40
7.CD30
8.TRAF (s)
9.CASPASE 8
10.TNF
Now it is established that lymphoma are due to a critical event causing Heavy chain IgH to be transposed into proximity with a promoter of PAX5 gene leading to 2 events
1. stimulation of TLE4 that force differentiation to lymphocyte proliferation. TLE4 is a tranduction enhancer which tell the cell no Brain differentiation by a negative regulation of the Groucho family members! but it does force proliferation of lymphoblasts forward.
2.to insure survival of lymphoblasts, it consumes or de-repress the Death protein 6 leaving FAS intact and therefore NO APOPTOSIS, and favoring an intact Kinechore plate to preserve speedy cell division by preserving the Centromere protein C. Decrease of the Death receptor has a significant 3rd effect on a series of TNF receptors and ligand which ultimately through ETS1 and TTRAP end up affecting CD40 leading to weakening of the cell presentation of Antigen which is the underlying immunodeficeinec in lymphoma (similar to hyper IgM Syndrome)
This is the underlying pathophysiology of Lymphoma as it looks today.
OF NOTE CD40 IS ALSO INVOLVED IN ALZHEIMER!
JUST REMEMBER THE CD40 THRU TRAF GENES INTERACTS WITH CD30 TO ACTIVATE THE NF-kB AND CYCLINS!
CRITICAL TARGET IN THIS PATHWAYS
1.PROTEIN QD
2,KINETOCHORE, CENTROMERE PROTEIN C
3.FAS
4.DEATH PROTEIN 6
5.TTRAP
6.CD40
7.CD30
8.TRAF (s)
9.CASPASE 8
10.TNF
AURKA GENE/ AN INDICATION FOR TAXANES?
"Aurora A is a member of a family of mitotic serine/threonine kinases. It is implicated with important processes during mitosis and meiosis whose proper function is integral for healthy cell proliferation. Aurora A is activated by one or more phosphorylations[4] and its activity peaks during the G2 phase to M phase transition in the cell cycle.[5]"WIKIPEDIA
"Aurora A is critical for proper formation of mitotic spindle. It is required for the recruitment of several different proteins important to the spindle formation. Among these target proteins are TACC, a microtubule-associated protein that stabilizes centrosomal microtubules and Kinesin 5, a motor protein involved in the formation of the bipolar mitotic spindle.[4] γ-tubulins, the base structure from which centrosomal microtubules polymerize, are also recruited by Aurora A. Without Aurora A the centrosome does not accumulate the quantity of γ-tubulin that normal centrosomes recruit prior to entering anaphase. Though the cell cycle continues even in the absence of deficient γ-tubulin, the centrosome never fully matures; it organizes fewer aster microtubules than normal.[5]"WIKIPEDIA
"
Aurora A dysregulation has been associated with high occurrence of cancer. For example, one study showed over-expression of Aurora A in 94 percent of the invasive tissue growth in breast cancer, while surrounding, healthy tissues had normal levels of Aurora A expression.[4] Dysregulation of Aurora A may lead to cancer because Aurora A is required for the completion of cytokinesis. If the cell begins mitosis, duplicates its DNA, but is then not able to divide into two separate cells it becomes an aneuploid- containing more chromosomes than normal. Aneuploidy is a trait of many cancerous tumors.[7] Ordinarily, Aurora A expression levels are kept in check by the tumor suppressor protein p53.[4]
Mutations of the chromosome region that contains Aurora A, 20q13, are generally considered to have a poor prognosis.[4]WIKIPEDIA"
===============================================================
AGENTS THAT ACTIVATES P53 WILL HAVE A STRONGER EFFECT IN THESE CANCER AS THEY WILL SUPPRESS THE AURORA A
AND SHOULD MORE LIKELY COMBINED TO TAXANE AS IT ACTS ON MICROTUBULES?
"Aurora A is a member of a family of mitotic serine/threonine kinases. It is implicated with important processes during mitosis and meiosis whose proper function is integral for healthy cell proliferation. Aurora A is activated by one or more phosphorylations[4] and its activity peaks during the G2 phase to M phase transition in the cell cycle.[5]"WIKIPEDIA
"Aurora A is critical for proper formation of mitotic spindle. It is required for the recruitment of several different proteins important to the spindle formation. Among these target proteins are TACC, a microtubule-associated protein that stabilizes centrosomal microtubules and Kinesin 5, a motor protein involved in the formation of the bipolar mitotic spindle.[4] γ-tubulins, the base structure from which centrosomal microtubules polymerize, are also recruited by Aurora A. Without Aurora A the centrosome does not accumulate the quantity of γ-tubulin that normal centrosomes recruit prior to entering anaphase. Though the cell cycle continues even in the absence of deficient γ-tubulin, the centrosome never fully matures; it organizes fewer aster microtubules than normal.[5]"WIKIPEDIA
"
Aurora A dysregulation has been associated with high occurrence of cancer. For example, one study showed over-expression of Aurora A in 94 percent of the invasive tissue growth in breast cancer, while surrounding, healthy tissues had normal levels of Aurora A expression.[4] Dysregulation of Aurora A may lead to cancer because Aurora A is required for the completion of cytokinesis. If the cell begins mitosis, duplicates its DNA, but is then not able to divide into two separate cells it becomes an aneuploid- containing more chromosomes than normal. Aneuploidy is a trait of many cancerous tumors.[7] Ordinarily, Aurora A expression levels are kept in check by the tumor suppressor protein p53.[4]
Mutations of the chromosome region that contains Aurora A, 20q13, are generally considered to have a poor prognosis.[4]WIKIPEDIA"
===============================================================
AGENTS THAT ACTIVATES P53 WILL HAVE A STRONGER EFFECT IN THESE CANCER AS THEY WILL SUPPRESS THE AURORA A
AND SHOULD MORE LIKELY COMBINED TO TAXANE AS IT ACTS ON MICROTUBULES?
OTHER GENES IN BREAST CANCER/ and questions at CRBCM
1-RRM2
A ribonucleotide reductase transforming RNA to DNA,
Zang et al "Overexpression of RRM2 significantly enhances the invasive and metastatic potential of tumor. Angiogenesis is critical to tumor malignancy; it plays an essential role in tumor growth and metastasis...the angiogenic potential of tumor is affected by RRM2."
==============================================================
how does RRM2B mutation affect the MTOR inhibitors?
is this the marker for use of Irinotecan
Is MMP9 informative (prognosis) in patient on CPT-11
what is the frequency of RRM2 Mutations in Ovarian cancers?
WHEN IS THE LAST TIME YOU SED TOPOTECAN AND IRINOTECAN IN BREAST CANCER? DOES MUTATION IN RRM2 CONVINCE YOU TO TRY IT?
1-RRM2
A ribonucleotide reductase transforming RNA to DNA,
Zang et al "Overexpression of RRM2 significantly enhances the invasive and metastatic potential of tumor. Angiogenesis is critical to tumor malignancy; it plays an essential role in tumor growth and metastasis...the angiogenic potential of tumor is affected by RRM2."
Overexpression of RRM2 decreases thrombspondin-1 and increases VEGF production in human cancer cells in vitro and in vivo: implication of RRM2 in angiogenesis.
Boukovinas et al
" Overexpression of RRM1 and RRM2 has been associated with gemcitabine resistance. BRCA1 overexpression increases sensitivity to paclitaxel and docetaxel. "
Duxbury MS, Whang EE (2007). "RRM2 induces NF-kappaB-dependent MMP-9 activation and enhances cellular invasiveness". Biochem. Biophys. Res. Commun. 354 (1): 190–6.
Zhang:" Silencing of RRM1 and RRM2, which encode the large and small subunits of the human ribonucleotide reductase complex, respectively, markedly enhanced the cytotoxicity of the topoisomerase I inhibitor camptothecin (CPT). Silencing of RRM2 was also found to enhance DNA damage as measured by histone γ-H2AX. Further studies showed that CPT up-regulates both RRM1 and RRM2 mRNA and protein levels and induces the nuclear translocation of RRM2. The checkpoint kinase 1 (Chk1) was up-regulated and activated in response to CPT, and CHEK1 down-regulation by siRNA and small molecule inhibitors of Chk1 blocked RRM2 induction by CPT. CHEK1 siRNA also suppressed E2F1 up-regulation by CPT, and silencing of E2F1 suppressed the up-regulation of RRM2. Silencing of ATR or ATM and inhibition of ATM activity by KU-55933 blocked Chk1 activation and RRM2 up-regulation. This study links the known components of CPT-induced DNA damage response with proteins required for the synthesis of dNTPs and DNA repair. Specifically, we propose that upon DNA damage, Chk1 activation, mediated by ATM and ATR, up-regulates RRM2 expression through the E2F1 transcription factor. Up-regulation in RRM2 expression levels coupled with its nuclear recruitment suggests an active role for ribonucleotide reductase in the cellular response to CPT-mediated DNA damage that could potentially be exploited as a strategy for enhancing the efficacy of topoisomerase I inhibitors.Two water-soluble DNA topoisomerase 1 (Top1)2 inhibitors, derived from camptothecin (CPT), are in clinical use; topotecan, for the treatment of ovarian and lung cancers, and irinotecan, for colorectal cancers."
Mutation of RRM2B, encoding p53-controlled ribonucleotide reductase (p53R2), causes severe mitochondrial DNA depletion
Alice Bourdon1"The mtDNA depletion triggered by p53R2 alterations in both human and mouse implies that p53R2 has a crucial role in dNTP supply for mtDNA synthesis."==============================================================
how does RRM2B mutation affect the MTOR inhibitors?
is this the marker for use of Irinotecan
Is MMP9 informative (prognosis) in patient on CPT-11
what is the frequency of RRM2 Mutations in Ovarian cancers?
WHEN IS THE LAST TIME YOU SED TOPOTECAN AND IRINOTECAN IN BREAST CANCER? DOES MUTATION IN RRM2 CONVINCE YOU TO TRY IT?
GI CANCERS UPDATE
*Following CEA every 3 months and CT once every year was compared to CT every 6 months -no difference in progression free survival or OS,
*Maintenance Xeloda and Avastin in Metastatic Colorectal cancer? read CAIRO3 by the Dutch!
*"An interesting negative study from Charles Loprinzi and his colleagues[4] looked at the impact of infused magnesium and calcium on the prevention of the sensory neurotoxicity associated with oxaliplatin and found no benefit. This was a large, well-designed study that leaves that myth to rest, which may be of benefit in some way." DAVID KERR.
*Abraxane -gemzar a new standard in metastatic pancreatic cancer!
*Following CEA every 3 months and CT once every year was compared to CT every 6 months -no difference in progression free survival or OS,
*Maintenance Xeloda and Avastin in Metastatic Colorectal cancer? read CAIRO3 by the Dutch!
*"An interesting negative study from Charles Loprinzi and his colleagues[4] looked at the impact of infused magnesium and calcium on the prevention of the sensory neurotoxicity associated with oxaliplatin and found no benefit. This was a large, well-designed study that leaves that myth to rest, which may be of benefit in some way." DAVID KERR.
*Abraxane -gemzar a new standard in metastatic pancreatic cancer!
ACTIVITIES AT CRBCM
We promised to submit an RFA at NIH yesterday, little did we know how tricky the process is. The process is so tricky you wonder if it is designed to discourage the novice. We failed in our attempt. But we are a Coalition fighting for a just cause, we do not give-up. We are going back right at it as sunrise comes tomorrow because we have to keep fighting until the resistances are brought down, until they hear our voice and/or until the enemy becomes irrelevant!
The process of filling up the the application is full of exasperating moments made for you to quit. We understood quickly that after filling up one answer, you have to give time to the computer to process the full file with the new information and save the document! Literally, after each input you got to save the full file otherwise you lose it all every 2-3 minutes! For the makers of the Internet, the government has ZERO for computer "practicality and easiness" of their program. The RED TAPE is in full display. You need numbers to make Albert Einstein think again! You need EIN, DUNS,NUNS,SAMS AND CAGE,ERA commons, and the surprise wait for you when you want to submit, there and you will MPIN. Why all these and why wait until the end at submission, just to frustrate the hell out of you: "you are not the AOR". The all purpose is to frustrate applicants. And get this, when we called them, they do not have either your DUNS which they gave you, and that you need to access the SAMS. And they try to help you but can't because you do not have the same "options on the screen". Your screen offer you less options then theirs! The government does not give you the options to register by your self for the MPIN. They select who they give it to! So we could not file. Tommorrow, we are back at it! try it but take some Xanax first!
ONE THING THEY HAVE TO REALIZE, WE WILL NOT QUIT THE FIGHT!
We promised to submit an RFA at NIH yesterday, little did we know how tricky the process is. The process is so tricky you wonder if it is designed to discourage the novice. We failed in our attempt. But we are a Coalition fighting for a just cause, we do not give-up. We are going back right at it as sunrise comes tomorrow because we have to keep fighting until the resistances are brought down, until they hear our voice and/or until the enemy becomes irrelevant!
The process of filling up the the application is full of exasperating moments made for you to quit. We understood quickly that after filling up one answer, you have to give time to the computer to process the full file with the new information and save the document! Literally, after each input you got to save the full file otherwise you lose it all every 2-3 minutes! For the makers of the Internet, the government has ZERO for computer "practicality and easiness" of their program. The RED TAPE is in full display. You need numbers to make Albert Einstein think again! You need EIN, DUNS,NUNS,SAMS AND CAGE,ERA commons, and the surprise wait for you when you want to submit, there and you will MPIN. Why all these and why wait until the end at submission, just to frustrate the hell out of you: "you are not the AOR". The all purpose is to frustrate applicants. And get this, when we called them, they do not have either your DUNS which they gave you, and that you need to access the SAMS. And they try to help you but can't because you do not have the same "options on the screen". Your screen offer you less options then theirs! The government does not give you the options to register by your self for the MPIN. They select who they give it to! So we could not file. Tommorrow, we are back at it! try it but take some Xanax first!
ONE THING THEY HAVE TO REALIZE, WE WILL NOT QUIT THE FIGHT!
Thursday, June 20, 2013
DON'T RESIST, JUST GO TO ARTICLE! IT WILL SET YOU FREE AND MAKE YOU AWARE,
This coverage is not sanctioned by, nor a part of, the American Medical Association.
CHICAGO — Physicians voted overwhelmingly to
label obesity as a disease that requires a range of interventions to
advance treatment and prevention.
Medscape Medical News from the:
QUESTION BEFORE CPRIT!
"However, some important context is missing even as legitimate questions are asked. We have heard a lot over the last 12 months about the three questionable grants which CPRIT awarded. But we haven’t heard much about the other 498 grants issued by the agency since it started operating in 2009. The headlines leave out the tremendous impact $835 million in CPRIT grant dollars have made in Texas on cancer prevention, research and product development during the past three years."The LIVESTRONG Foundation.
A FULL ASSESSMENT IS DUE,
HOW THESE ACHIEVEMENTS FIT WITHIN THE DREAM FOR CANCER CURE PLAN! IS JUST ANOTHER QUESTION.
"However, some important context is missing even as legitimate questions are asked. We have heard a lot over the last 12 months about the three questionable grants which CPRIT awarded. But we haven’t heard much about the other 498 grants issued by the agency since it started operating in 2009. The headlines leave out the tremendous impact $835 million in CPRIT grant dollars have made in Texas on cancer prevention, research and product development during the past three years."The LIVESTRONG Foundation.
A FULL ASSESSMENT IS DUE,
HOW THESE ACHIEVEMENTS FIT WITHIN THE DREAM FOR CANCER CURE PLAN! IS JUST ANOTHER QUESTION.
ASK YOURSELF, CAN YOU USE OF ANTI-TNF AND OR MACROLIDE IN COMBINATION OF ANTI-VIRAL ENOUGH TO CUT DOWN MORTALITY DURING A FLU EPIDEMIC?
AND WHILE YOU ARE ASKING YOURSELF THIS, I AM WRITING AN RFA FOR NCI.
The assumption here is that death from the flu is directly linked to TNF secretion and cyclins secretions under activation of the NF-kB, and these 2 drugs can tamper these 2 components/PATHWAYS. check it out!
================================================================
ROLE OF BAFETINIB IN CMML AND MONOCYTIC LEUKEMIA
FOLLOWING THE LYN GENE DANCE!
SHOULD GLIMEPIRIDE BE CONTRE-INDICATED IN THESE DISEASES BECAUSE IT IS A LYN ACTIVATOR?
ACTIVATION OF LYN LEADS TO ACTIVATION OF INPP5D WHICH IS DEFINED AS FOLLOW: "Overall, the protein functions as a negative regulator of myeliod cell proliferation and survival. Alternate transcriptional splice variants, encoding different isoforms, have been characterized.[" AND THEREFORE A LEGITIMATE TARGET FOR THERAPY!
AND WHILE YOU ARE ASKING YOURSELF THIS, I AM WRITING AN RFA FOR NCI.
The assumption here is that death from the flu is directly linked to TNF secretion and cyclins secretions under activation of the NF-kB, and these 2 drugs can tamper these 2 components/PATHWAYS. check it out!
================================================================
ROLE OF BAFETINIB IN CMML AND MONOCYTIC LEUKEMIA
FOLLOWING THE LYN GENE DANCE!
SHOULD GLIMEPIRIDE BE CONTRE-INDICATED IN THESE DISEASES BECAUSE IT IS A LYN ACTIVATOR?
ACTIVATION OF LYN LEADS TO ACTIVATION OF INPP5D WHICH IS DEFINED AS FOLLOW: "Overall, the protein functions as a negative regulator of myeliod cell proliferation and survival. Alternate transcriptional splice variants, encoding different isoforms, have been characterized.[" AND THEREFORE A LEGITIMATE TARGET FOR THERAPY!
COMING THROUGH MEDSCAPE!
*35% of paroxysmal nocturnal hemoglobinuria (PNH) patients die within 5 years of diagnosis1 Three experts discuss how the pathophysiology of PNH can manifest in life-threatening complications such as chronic kidney disease, pulmonary hypertension, and thrombosis.
*GO TOARTICLE
Association of metformin use with cancer incidence and mortality: A meta-analysis
Zhang P et al. – Metformin can reduce the incidence of
overall cancer, liver cancer, pancreatic cancer, colorectal cancer and
breast cancer as well as the mortality of overall cancer, liver cancer
and breast cancer. No beneficial effect on prostate cancer incidence was
found for meformin intake in the meta-analysis."
*GO TO ARTICLE!
The US Food and Drug Administration (FDA) is
investigating 2 unexplained deaths of patients who received an
intramuscular injection of the antipsychotic drug olanzapine pamoate (Zyprexa Relprevv, Eli Lilly).
*35% of paroxysmal nocturnal hemoglobinuria (PNH) patients die within 5 years of diagnosis1 Three experts discuss how the pathophysiology of PNH can manifest in life-threatening complications such as chronic kidney disease, pulmonary hypertension, and thrombosis.
*GO TOARTICLE
Association of metformin use with cancer incidence and mortality: A meta-analysis
Cancer Epidemiology, 04/16/2013
Review Article
*GO TO ARTICLE!
FDA Investigates 2 Deaths Involving Olanzapine
Mark Crane
Jun 18, 2013
Editors' Recommendations
Wednesday, June 19, 2013

What is Smarter Screening?
High throughput
screening (HTS), or the process by which libraries of small molecule
compounds are individually assessed for binding, activating or
inactivating biological activity in drug target molecules, has been part
of the drug discovery process for more than two decades. Its primary
purpose is to rapidly select which compounds possess the desired
activity and thereby undergo further testing and optimization. Since its
inception, HTS methods have undergone significant change: the early
dependence on “home-brewed” assay chemistries and basic research
instruments evolved into industrialized methods incorporating millions
of compounds that could be screened against purified drug targets using
sophisticated liquid handling, detection and robotics.
In this Application
Guide, we describe key liquid handling and detection instrumentation
for HTS methods and provide four HTS case studies. The case studies use
commercially available compound libraries and assay chemistries suitable
for nuclear receptor, GPCR, cellular kinase, as well as epigenetic drug
targets.
This Application Guide covers:
- Androgen Receptor Agonist/Antagonist Studies using the Cell-based Human AR Reporter Assay System from INDIGO Biosciences
- GLP-1 Ligand Binding Analysis using a Cell-Based HTRF® Tag-lite® Assay
- Quantification of Endogenous Cellular Kinase Activity using a Cell-Based HTRF Phospho-STAT3 (Tyr705) Protein Kinase Assay
- Assessment of Histone Deacetylase 1 Inhibition using the Bioluminescent HDAC-Glo™ I/II Assay
Tag-lite and HTRF are registered trademarks of Cisbio Bioassays.
HDAC-Glo is a trademark of Promega Corporation.

Sign up to win a 405™ Touch Microplate Washer with Verify™ Technology for your lab!
A
random drawing from all eligible entries will be completed on Tuesday,
July 2nd, 2013. The lucky winner will be notified immediately.
Decades of BioTek’s
automated microplate washing expertise culminates in the 405 Touch and
we want you to experience this latest technology in your lab… so we’re
giving one away! Win a BioTek 405 Touch with NEW Verify Technology…
BioTek’s flagship microplate washer. See why thousands of labs choose
BioTek’s 405 for their microplate washing needs!
*EP 1 637 887
Learn more about 405 Touch with Verify
Features
Applications
Contest Terms
- Valid April 1, 2013 through June 30, 2013.
- Only one entry per person will be accepted.
- One winner will be randomly drawn from all entries.
- The model to be given away is 405TSUSQ. No exceptions, no substitutions.
- The winner will also receive a free Complete Dispense/Waste System 115V/230V, 4L bottles, p/n 1170530. No exceptions, no substitutions.
- BioTek employees and distributors are not eligible for this contest.
GO TO ARTICLE OF INTEREST
6 months versus 12 months of adjuvant trastuzumab for patients
with HER2-positive early breast cancer (PHARE): a randomised phase 3
trial
Pivot X et al. – After 3·5 years follow-up, we failed to
show that 6 months of treatment with trastuzumab was non-inferior to 12
months of trastuzumab. Despite the higher rates of cardiac events, 12
months of adjuvant trastuzmab should remain the standard of care.
GO TO ARTICLES OF INTEREST
===================================================
AFTER FAILURE OF AVASTIN, THE TUMOR IS READY FOR MTOR INHIBITOR
Phase ii study of everolimus in patients with metastatic
colorectal adenocarcinoma previously treated with bevacizumab-,
fluoropyrimidine-, oxaliplatin-, and irinotecan-based regimens
Ng K et al. – Everolimus 70 mg/week or 10 mg/day was well
tolerated but did not confer meaningful efficacy in heavily pretreated
mCRC patients.
GO TO ARTICLE
=====================================================
USEFUL TIPR FOR PRACTITIONER!
Lactate dehydrogenase B: a metabolic marker of response to neoadjuvant chemotherapy in breast cancer
Dennison JB et al. – Expression of LDHB predicted response
to neoadjuvant chemotherapy within clinical subtypes independently of
standard prognostic markers and PAM50 subtyping. These observations
support prospective clinical evaluation of LDHB as a predictive marker
of response for patients with breast cancer receiving neoadjuvant
chemotherapy.
GO TO ARTICLE!
======================================= I AM GOING :HMMMMM, SOME RESEARCHER ARE STILL WORKING AT THIS NOW?
One year of adjuvant tamoxifen compared with chemotherapy and tamoxifen in postmenopausal patients with stage II breast cancer
Ejlertsen B et al. – CMF added to 1year of tamoxifen
reduces the risk of a DFS event. The benefit from CMF was not
significantly different in Luminal A and B subtypes.
GO TO ARTICLE!
6 months versus 12 months of adjuvant trastuzumab for patients
with HER2-positive early breast cancer (PHARE): a randomised phase 3
trial
The Lancet Oncology, 06/12/2013
Clinical Article
GO TO ARTICLES OF INTEREST
===================================================
AFTER FAILURE OF AVASTIN, THE TUMOR IS READY FOR MTOR INHIBITOR
Phase ii study of everolimus in patients with metastatic
colorectal adenocarcinoma previously treated with bevacizumab-,
fluoropyrimidine-, oxaliplatin-, and irinotecan-based regimens
Clinical Cancer Research, 06/11/2013
Clinical Article
GO TO ARTICLE
=====================================================
USEFUL TIPR FOR PRACTITIONER!
Lactate dehydrogenase B: a metabolic marker of response to neoadjuvant chemotherapy in breast cancer
Clinical Cancer Research, 06/13/2013
GO TO ARTICLE!
======================================= I AM GOING :HMMMMM, SOME RESEARCHER ARE STILL WORKING AT THIS NOW?
One year of adjuvant tamoxifen compared with chemotherapy and tamoxifen in postmenopausal patients with stage II breast cancer
European Journal of Cancer, 06/13/2013
Clinical Article
GO TO ARTICLE!
CPRIT AND THE CURE OF CANCER
As it is preparing to relaunch its funding of cancer research and prevention, it is imperative that its leaders realize that external influences have realized its opulence and weaknesses, and are ready to seduce and suck it of all its juices once more with pretentious programs that may fit universities'objectives but not CPRIT's.
The previous experience is summarized like this, almost 1 billion went to 72 institutions instead of 200 (or more) and nothing tangible really to show for! Obsession with funding universities without creating real competition had created a sens of entitlement and real production of innovation is still lacking! Many institutions had pulled old projects from their past and submitted them for funding visiting old paths to known outcome! Sold findings and patents were refunded and relocated to Texas without shame, wasting resources. The idea that we promised to fund this and that has now stolen the power to reexamined projects as to their true validity. CPRIT needs to regroup, recapture its soul, and focus on the cure.
The difference between development and lack of it is man made, Africa is where it is not because of lack of potentials but because of lack of understanding by humans shackled by traditional practices and mis-guided understanding of currents systems. The rise of China and some prominent countries is a demonstration that vision and focus can lead to success of different shape. What is lacking here is an understanding of what cure should look like which will allow to pick and choose projects of relevant importance that will show advances toward a cure as it looks to CPRIT. Lack of this type of focus, will not only lead to waste and open to political games, distractions and false tendencies! It is imperative that CPRIT recaptures its soul, knows that as a source of funding, it is the independent decider of how the game should be played, and where to lead this boat. It should be mindful that all Texas is funding this, and its actions should touch the life of Texans as a whole not just few cities. Its meeting should show a spirit of freedom when it comes to deciding where to place money. Same beneficiaries at each meeting leads to waste of resources and fails to show respect to the whole Texans while reducing the pool of solicited imagination. Cancer cure may not come from the standard suspects! Small incremental progress may!
There are Cities like El Paso that has been ignored all together despite the existence of brave researchers here. 72 beneficiaries of 1 billions dollars is a club! Not a true competition! The Number of participants matter because the more they are, the more they can attract other federal funding to Texas! CPRIT wants to be richer by finding new target therapies, but creating various viable institutions that could potentially apply for other grants is an ignored potential for true revenues today (not tomorrow or by chance!). It takes vision to see this!
The bringing in of an oncologist on board will allow to not only define a cure better, but put CPRIT at the edge of progress and get a feedback from Doctors as to where the interest is today in Oncology practice! Not all bright researcher idea is well received by practitioners who are the first to handle side effects in patients!
We have seen the expressed will to comply with recommendations from the legislature, what is left to be seen is if there is indeed a soul, vision and courage to CPRIT and its leaders!
As it is preparing to relaunch its funding of cancer research and prevention, it is imperative that its leaders realize that external influences have realized its opulence and weaknesses, and are ready to seduce and suck it of all its juices once more with pretentious programs that may fit universities'objectives but not CPRIT's.
The previous experience is summarized like this, almost 1 billion went to 72 institutions instead of 200 (or more) and nothing tangible really to show for! Obsession with funding universities without creating real competition had created a sens of entitlement and real production of innovation is still lacking! Many institutions had pulled old projects from their past and submitted them for funding visiting old paths to known outcome! Sold findings and patents were refunded and relocated to Texas without shame, wasting resources. The idea that we promised to fund this and that has now stolen the power to reexamined projects as to their true validity. CPRIT needs to regroup, recapture its soul, and focus on the cure.
The difference between development and lack of it is man made, Africa is where it is not because of lack of potentials but because of lack of understanding by humans shackled by traditional practices and mis-guided understanding of currents systems. The rise of China and some prominent countries is a demonstration that vision and focus can lead to success of different shape. What is lacking here is an understanding of what cure should look like which will allow to pick and choose projects of relevant importance that will show advances toward a cure as it looks to CPRIT. Lack of this type of focus, will not only lead to waste and open to political games, distractions and false tendencies! It is imperative that CPRIT recaptures its soul, knows that as a source of funding, it is the independent decider of how the game should be played, and where to lead this boat. It should be mindful that all Texas is funding this, and its actions should touch the life of Texans as a whole not just few cities. Its meeting should show a spirit of freedom when it comes to deciding where to place money. Same beneficiaries at each meeting leads to waste of resources and fails to show respect to the whole Texans while reducing the pool of solicited imagination. Cancer cure may not come from the standard suspects! Small incremental progress may!
There are Cities like El Paso that has been ignored all together despite the existence of brave researchers here. 72 beneficiaries of 1 billions dollars is a club! Not a true competition! The Number of participants matter because the more they are, the more they can attract other federal funding to Texas! CPRIT wants to be richer by finding new target therapies, but creating various viable institutions that could potentially apply for other grants is an ignored potential for true revenues today (not tomorrow or by chance!). It takes vision to see this!
The bringing in of an oncologist on board will allow to not only define a cure better, but put CPRIT at the edge of progress and get a feedback from Doctors as to where the interest is today in Oncology practice! Not all bright researcher idea is well received by practitioners who are the first to handle side effects in patients!
We have seen the expressed will to comply with recommendations from the legislature, what is left to be seen is if there is indeed a soul, vision and courage to CPRIT and its leaders!
Tuesday, June 18, 2013
CPRIT/ GONE FOR THE SECOND ROUND! WISH THEY WILL DO THE RIGHT THING NOW! BUT ONLY HISTORY WILL TELL!
Governor Perry has signed into law Senate Bill 149, the reform bill revising CPRIT’s enabling legislation. We appreciate the leadership and vision of the Legislature and Governor Perry to pass this important measure. The legislation strengthens CPRIT and allows the agency to fulfill its vital mission to fight cancer in Texas with transparency and accountability. The CPRIT staff has already begun to implement the reforms contained in SB 149, and once authorized to do so by state leadership, will restart our grant-making processes.
=======================================================
INSTITUTIONS, LIKE PEOPLE ARE JUDGED BY WHAT THEY DO, NOT BY WHAT THEY PROMISE. CAUSE THOSE WHO RUINED IT FIRST TIME ARE STILL STANDING, AND READY TO TAKE IT AGAIN FIR THE 2 BILLIONS REMAINING...LIKE THE SENATORS WHO WANT FINE CONTROL, THEY HAVE SHARPEN THEIR TACTICS AND DEVISE BETTER WAYS TO MAKE IT LEGIT! PHONE CALLS HAVE BEEN MADE TO READY INSIDERS, FOR A NEW BALL DANCE ONCE MORE. ALL WE HAVE TO PRAY FOR, THAT ONE LEADER WILL RISE TO GIVE THE REST OF US A CHANCE ! CPRIT IS STILL A PROMISE WITH FULL POTENTIAL TO BE REALIZED! WILL IT?
Governor Perry has signed into law Senate Bill 149, the reform bill revising CPRIT’s enabling legislation. We appreciate the leadership and vision of the Legislature and Governor Perry to pass this important measure. The legislation strengthens CPRIT and allows the agency to fulfill its vital mission to fight cancer in Texas with transparency and accountability. The CPRIT staff has already begun to implement the reforms contained in SB 149, and once authorized to do so by state leadership, will restart our grant-making processes.
=======================================================
INSTITUTIONS, LIKE PEOPLE ARE JUDGED BY WHAT THEY DO, NOT BY WHAT THEY PROMISE. CAUSE THOSE WHO RUINED IT FIRST TIME ARE STILL STANDING, AND READY TO TAKE IT AGAIN FIR THE 2 BILLIONS REMAINING...LIKE THE SENATORS WHO WANT FINE CONTROL, THEY HAVE SHARPEN THEIR TACTICS AND DEVISE BETTER WAYS TO MAKE IT LEGIT! PHONE CALLS HAVE BEEN MADE TO READY INSIDERS, FOR A NEW BALL DANCE ONCE MORE. ALL WE HAVE TO PRAY FOR, THAT ONE LEADER WILL RISE TO GIVE THE REST OF US A CHANCE ! CPRIT IS STILL A PROMISE WITH FULL POTENTIAL TO BE REALIZED! WILL IT?
WHEN IT WORKS, GIVE IT FOR 3 YEARS!
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Subscribe to:
Posts (Atom)