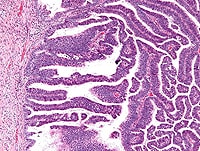
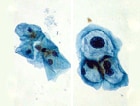
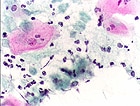
Cervical Screening Guidelines Updated
Guidelines posted online
March 21 by the American Society for Colposcopy and Cervical Pathology
(ASCCP) now address management of discordant co-tests, in which results
of either Papanicolaou (Pap) smear or human papillomavirus (HPV) testing
are positive, but not both. The new algorithms update the 2006
recommendations, based on risk analysis of new data from nearly 1.4
million women in a National Cancer Institute–Kaiser Permanente Northern
California cohort.
"The primary focus of the revised recommendations [is] the
obsolescence of the 2006 guidelines and the fact that guidelines on
management of women with Pap tests read as unsatisfactory or missing
endocervical cells were never ratified by a consensus conference," lead
author L. Stewart Massad Jr, MD, professor of obstetrics and gynecology
at Washington University in St. Louis, Missouri, and ASCCP board of
directors member, told Medscape Medical News by email.
According to the 2012 consensus guidelines from the American Cancer Society, ASCCP, and the American Society of Clinical Pathologists, women aged 21 to 65 years should have Pap tests every 3 years. Further screening is not recommended for women with test results negative for precancerous lesions.
"Women ages 21-24 have a very low risk of cancer," Dr. Massad said. "Many lesions identified by Pap testing in this group are HPV-related changes that will resolve without treatment as the body's immune system recognizes and reacts to the HPV; they are not precancer. Treatment of these lesions with usual destructive therapies for precancer can predispose to preterm delivery if these young women later conceive."
For women aged 30 to 64 years, the Pap test and HPV test are preferred for screening. Although previous guidelines had recommended return to "routine screening" after evaluation, it was unknown whether that was still acceptable given the longer screening intervals.
Algorithms in the updated guidelines include the following:
According to the 2012 consensus guidelines from the American Cancer Society, ASCCP, and the American Society of Clinical Pathologists, women aged 21 to 65 years should have Pap tests every 3 years. Further screening is not recommended for women with test results negative for precancerous lesions.
"Women ages 21-24 have a very low risk of cancer," Dr. Massad said. "Many lesions identified by Pap testing in this group are HPV-related changes that will resolve without treatment as the body's immune system recognizes and reacts to the HPV; they are not precancer. Treatment of these lesions with usual destructive therapies for precancer can predispose to preterm delivery if these young women later conceive."
For women aged 30 to 64 years, the Pap test and HPV test are preferred for screening. Although previous guidelines had recommended return to "routine screening" after evaluation, it was unknown whether that was still acceptable given the longer screening intervals.
Algorithms in the updated guidelines include the following:
OC468900PROF-D 10/12
Information from Industry
- Management of discordant co-tests, in which results of either Pap smear or HPV testing are positive, but not both, with integration of co-testing into follow-up. Colposcopy and/or HPV DNA typing may be indicated.
- Return to "routine" screening in women treated for cervical cancer.
- Extension of management guidelines for adolescents under 21 years of age to women under 25 years of age. Workup varies according to findings of atypical squamous cells of undetermined significance, or low-grade or high-grade squamous intraepithelial lesion, and may include colposcopy.
- Consideration of whether cervical intraepithelial neoplasia grade 1 (CIN1) on endocervical canal curettage (ECC) should be treated as positive ECC or CIN1.
- Management of women with unsatisfactory cytologic findings and specimens that are missing endocervical or transformation zone components. Colposcopy may be required for women with positive HPV results or with repeated unsatisfactory cytologic findings.