-
Anonymous
Posted January 28, 2013Heard all sorts of things about Simba Beer mentioned in the stor
Heard all sorts of things about Simba Beer mentioned in the story. Does it still exist? How does it taste?Was this review helpful? Yes No Report this review
Wish I could try the traditional chicken and the peanut paste snack! -
KONGOKONGAPosted January 28, 2013
Just wish to go on such a river journey, for days on end, seeing
Just wish to go on such a river journey, for days on end, seeing the lush forest pass by... vivid descriptions, the characters of the novel are well sketched and quickly feel like friends...Was this review helpful? Yes No Report this review
A blog about research, awareness, prevention, treatment and survivorship of Breast Cancer and all cancers, including targeted scientific research and a grassroots approach to increase screening for cancer, especially in the low income and under-insured population of El Paso, Texas, with a view to expand this new health care model to many other 'minority' populations across the United States and beyond
Sunday, February 3, 2013
FEW FACTS/10 news by HEMOnc today
1. BMI (body mass index) did not influence survival in early breast cancer women who received adhuvant therapy. This the work by EWERTZ et al. like any thing these day there was a trend toward benefit for those with BMI less than 30.
2. Reinfusion of Autologous T-cell containing a chimeric protein combining CD19 Antigen (CART 19 cells)
showed effective therapeutic effect in CLL.
3.Xarelto, a new anti-Xa was approved by the FDA in November 2012.
4. Adding Aspirin after discontinuation of Coumadin reduced recurrence rate of Thrombotic events.
5. BRAF inhibition and anti-CTLA4 Tremelimumab (+/- Interferon ) are the new kids on the Melanoma block! On the other spectrum, Taxol Carboplatin and Sorafenib failed to improve survival.
Trametinib may have activity in BRAF mutated Melanoma.
6. Zaltrap /Regorafenib has been approved in Metastatic Colorectal cancer. it is It is an anti-Angiogenesis agent by trapping VEGF A, B prior to its link to their respective receptors
7. Fibulin-3 an early biomarker for Mesothelioma? read the article...
8. Adding Avastin to Standard Taxane or Adriamycin based chemotherapy failed to improve survival in triple negative breast cancer as reported by Researcher in Scotland.
9. ZOLINZA (histone Deacetylase inhibitor ) may have a role in modulating Graft Vs Host (GVHD) in patients
10. Only a third of ITP patients who stopped Thrombopoietin Receptor Agonist (ELtrombopag) maintained a Platelet count 20-30,000 above the initial PLT count after 6 months of stopping the drug.
1. BMI (body mass index) did not influence survival in early breast cancer women who received adhuvant therapy. This the work by EWERTZ et al. like any thing these day there was a trend toward benefit for those with BMI less than 30.
2. Reinfusion of Autologous T-cell containing a chimeric protein combining CD19 Antigen (CART 19 cells)
showed effective therapeutic effect in CLL.
3.Xarelto, a new anti-Xa was approved by the FDA in November 2012.
4. Adding Aspirin after discontinuation of Coumadin reduced recurrence rate of Thrombotic events.
5. BRAF inhibition and anti-CTLA4 Tremelimumab (+/- Interferon ) are the new kids on the Melanoma block! On the other spectrum, Taxol Carboplatin and Sorafenib failed to improve survival.
Trametinib may have activity in BRAF mutated Melanoma.
6. Zaltrap /Regorafenib has been approved in Metastatic Colorectal cancer. it is It is an anti-Angiogenesis agent by trapping VEGF A, B prior to its link to their respective receptors
7. Fibulin-3 an early biomarker for Mesothelioma? read the article...
8. Adding Avastin to Standard Taxane or Adriamycin based chemotherapy failed to improve survival in triple negative breast cancer as reported by Researcher in Scotland.
9. ZOLINZA (histone Deacetylase inhibitor ) may have a role in modulating Graft Vs Host (GVHD) in patients
10. Only a third of ITP patients who stopped Thrombopoietin Receptor Agonist (ELtrombopag) maintained a Platelet count 20-30,000 above the initial PLT count after 6 months of stopping the drug.
Biotech firm credits CPRIT funds for experimental cancer-fighting drug
By: John A. Salazar

A 100-page report by the state auditor made public Monday slams the Cancer Research Institute of Texas, or CPRIT, with details of internal rule violations, misappropriated funds and grant applications not receiving proper scientific review.For now, all CPRIT grants have been put on hold at the direction of the governor's office. Following news of the State Audit report, a $25 Million CPRIT grant recipient shut down operations, causing 30 people to lose their job.
The audit is critical of how Statewide Clinical Trials Network of Texas was spending large portions of the grant.
The report is evidence of an ongoing statewide investigation of the five-year-old state agency. Widely celebrated upon being approved by Texas voters in 2007, CPRIT is now under criminal investigation after spending much of the past year in turmoil over questions surrounding several lucrative awards.
But scientists at local biotech research company Mirna Therapeutics says the CPRIT multimillion dollar grant it received is helping its battle against cancer.
The state’s Emerging Technology Fund gave $5 million to what scientists at the Mirna Therapeutics research lab call ‘pioneering research.’
The embattled CPRIT added another $10.3 million.
Mirna Therapeutics CEO Dr. Paul Lammers says the $15 million worth of public dollars has done its part inside his lab.
"Oh, absolutely vital, because it is very difficult time right now for funding in biotech," he said."Developing a new medicine, a new drug, doesn't matter if it's for cancer or for hypertension or diabetes, it is a long exercise.”
Scientists at Mirna have successfully created an experimental cocktail called the MRX34, which contains the mimic of a tumor suppressor called the miR-34. The miR-34 mimic can inhibit the growth of tumors related to lymphoma, liver, lung and prostate cancer. The drug is currently on its way to the U.S. Food Drug and Administration for clinical testing.
Lammers says the tumor suppressor wouldn’t have been created if it wasn’t for help from CPRIT funds.
“Beginning in 2008 was the first time we use that on animals where we saw tumors go away," Mirna Therapeutics Director of Research David Brown said.
Human clinical studies on MRX34 could begin in as early as two months.
The Associated Press contributed to this report.
=========================================================
SHARE
=============================================================
Comment:
We had warned CPRIT that some of these Biotech companies being bought or started-up will run off after the money dries out! What a waste! the ink is not even dried out...The FDA may not approve the so called advancement. And then?
A little more about HUMAN PAPILLOMA VIRUS AND CANCER:
--------------------------------------------------------------------------
HPV, the most sexually transmitted disease, has been implicated in:
- Cervical cancer
- Head and neck cancer
- Anal and most vaginal Cancers, penile cancers
- the role in lung and Esophageal cancers is not clear.
With over 130 types, only few have been implicated with cancer . (HPV16, 18 being the most cited since the work by Durst and Boshart)
90 % of infected people will see their infection cleared.
10 % will develop chronic infection. and by year 30 after original infection, 50% of the 10% will develop eventually a malignancy. It is widely believed that in those who developed the disease, the HPV gene E6 and E7 and their proteins products have managed to inactivate suppressor genes such as P53 and RB1 as part of the mechanism to cancerous transformation with loss of regulation of cellular proliferation, and control of chromosome instability. This process led to immortalization of Keratinocytes in the laboratory.
In the Oropharyngeal cancers, the verrucous squamous subtype is the most associated with HPV. HPV positive tumor however has a higher response rate with a better 2 year progression free disease.
Most of these cancers can be avoided by HPV immunization. The HPV presence can be confirmed by in-situ Hybridization. Expression of the P16 type appear to correlate with less P53 and RB1 inactivation and therefore leads to best prognosis whereas EGFR over expression tends to suggest a poorer outcome.
--------------------------------------------------------------------------
HPV, the most sexually transmitted disease, has been implicated in:
- Cervical cancer
- Head and neck cancer
- Anal and most vaginal Cancers, penile cancers
- the role in lung and Esophageal cancers is not clear.
With over 130 types, only few have been implicated with cancer . (HPV16, 18 being the most cited since the work by Durst and Boshart)
90 % of infected people will see their infection cleared.
10 % will develop chronic infection. and by year 30 after original infection, 50% of the 10% will develop eventually a malignancy. It is widely believed that in those who developed the disease, the HPV gene E6 and E7 and their proteins products have managed to inactivate suppressor genes such as P53 and RB1 as part of the mechanism to cancerous transformation with loss of regulation of cellular proliferation, and control of chromosome instability. This process led to immortalization of Keratinocytes in the laboratory.
In the Oropharyngeal cancers, the verrucous squamous subtype is the most associated with HPV. HPV positive tumor however has a higher response rate with a better 2 year progression free disease.
Most of these cancers can be avoided by HPV immunization. The HPV presence can be confirmed by in-situ Hybridization. Expression of the P16 type appear to correlate with less P53 and RB1 inactivation and therefore leads to best prognosis whereas EGFR over expression tends to suggest a poorer outcome.
HERBIMYCIN AND AVASTIN
Based of the evidence presented on the Myristoylation story, we predict that the combination of dasatinib or Herbimycin with Avastin will have a larger response rate on Angiosarcoma and papillary cancers because of a combined suppression of VGEF and SRC/MEK.
Dasatinib, previously known as BMS-354825, is a cancer drug produced by Bristol-Myers Squibb and sold under the trade name Sprycel. Dasatinib is an oral multi- BCR/ABL and Src family tyrosine kinase inhibitor approved for use in patients with chronic myelogenous leukemia (CML) after imatinib treatment and Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL). It is being evaluated for use in numerous other cancers, including advanced prostate cancer.
The drug is named after one of the inventor chemists, Jagabandhu Das, who was a member of the large discovery and development team at Bristol Myers Squibb.[1]
Herbimycin is a benzoquinone ansamycin antibiotic that binds to Hsp90
(Heat Shock Protein 90) and alters its function. HSP90 client proteins
play important roles in the regulation of the cell cycle, cell growth,
cell survival, apoptosis, angiogenesis and oncogenesis.
It was originally found by its herbicidal activity, and thus named.
-----------------------------------------------------------------------------------------------------------
Interferon will have a reasonable role, and studies have certainly reported cases of response of Angiosarcoma to Interferon. At CRBCM and the GREATER East cancer Center we are following a case of a young 38 year old female who has been referred to us after receiving Gemzar and Taxotere followed by MAID x 3 cycles for a 12 cm Angiosarcoma in the liver. She had an extensive skin reaction with desquamative feature to the Taxane. We plan to start her on Avastin alone at this point. We understand Navelbine could have a role.
Her case will be presented at Tumor Board to completely exhaust salvage surgical resection option. We are still awaiting a gene profiling which was not obtained at onset. An update will be forthcoming.
We are still working hard at CRBCM.
Role of Nexavar has also been discussed in Angiosarcoma...
Based of the evidence presented on the Myristoylation story, we predict that the combination of dasatinib or Herbimycin with Avastin will have a larger response rate on Angiosarcoma and papillary cancers because of a combined suppression of VGEF and SRC/MEK.
Dasatinib
From Wikipedia, the free encyclopedia
 |
|
|---|---|
| Systematic (IUPAC) name | |
| N-(2-chloro-6-methylphenyl)-2-[[6-[4-(2-hydroxyethyl)- 1-piperazinyl]-2-methyl-4-pyrimidinyl]amino]-5-thiazole carboxamide monohydrate |
|
| Clinical data | |
| Trade names | Sprycel |
| Pharmacokinetic data | |
| Protein binding | 96% |
| Metabolism | Hepatic |
| Half-life | 1.3 to 5 hours |
| Excretion | Fecal (85%), renal (4%) |
| Chemical data | |
| Formula | C22H26ClN7O2S |
The drug is named after one of the inventor chemists, Jagabandhu Das, who was a member of the large discovery and development team at Bristol Myers Squibb.[1]
Herbimycin
From Wikipedia, the free encyclopedia
Jump to: navigation, search
| Herbimycin | ||
|---|---|---|
 | ||
| Properties | ||
| Molecular formula | C30H42N2O9 | |
| Molar mass | 574.66 g/mol | |
| Hazards | ||
It was originally found by its herbicidal activity, and thus named.
Synonyms
- Antibiotic Tan 420F
- Herbimycin A
Biological activity
Herbimycin induces the degradation of proteins that are mutated in tumor cells such as v-Src, Bcr-Abl and p53 preferentially over their normal cellular counterparts. This effect is mediated via HSP90.-----------------------------------------------------------------------------------------------------------
Interferon will have a reasonable role, and studies have certainly reported cases of response of Angiosarcoma to Interferon. At CRBCM and the GREATER East cancer Center we are following a case of a young 38 year old female who has been referred to us after receiving Gemzar and Taxotere followed by MAID x 3 cycles for a 12 cm Angiosarcoma in the liver. She had an extensive skin reaction with desquamative feature to the Taxane. We plan to start her on Avastin alone at this point. We understand Navelbine could have a role.
Her case will be presented at Tumor Board to completely exhaust salvage surgical resection option. We are still awaiting a gene profiling which was not obtained at onset. An update will be forthcoming.
We are still working hard at CRBCM.
Role of Nexavar has also been discussed in Angiosarcoma...
Avastin versus Lucentis
BMJ
2012;
344
doi: http://dx.doi.org/10.1136/bmj.e3162
(Published 2 May 2012)
Cite this as:
BMJ
2012;344:e31
- Fiona Godlee, editor, BMJ
Drug
treatment for age related macular degeneration is one of medicine’s
success stories. As Ning Cheung and colleagues explain (doi:10.1136/bmj.e2970),
anti-vascular endothelial growth factors are preserving and restoring
vision to millions around the world. But a related and less edifying
story is stealing the limelight: ranibuzimab (Lucentis) versus
bevacizumab (Avastin). It casts a shadow over this great medical advance
and puts the world’s drug development and licensing systems under the
spotlight.
In its anti-cancer drug, bevacizumab, drug
developer Genentech has created what may be the world’s first “not me”
(as opposed to “me too”) drug, say Robert Campbell and colleagues (doi:10.1136/bmj.e2941).
Despite evidence that it works in macular degeneration, the
manufacturers and marketers (Roche in the US, Novartis in the UK and
elsewhere) are actively discouraging its use for this condition, even
going so far as taking legal action to prevent such off-label use. Why?
Because they want people to use their other drug, ranibuzimab, which is
licensed for treating macular degeneration.
The bottom
line is that ranibuzimab is about 12 times more expensive: Cheung and
colleagues report that the UK could save close to £300m (€368m; $485m) a
year if it were standard treatment. So are Roche and Novartis simply
fighting to protect their profits? They say no, that they are also
protecting patients from the cheaper drug’s higher risk profile.
Although data from the publicly funded CATT trial in the US found
similar effectiveness and safety for the two drugs in treating macular
degeneration, the safety of bevacizumab remains a worry. Concerns relate
to its greater systemic absorption and the fact that it has to be
decanted into smaller quantities for intraocular injection, which
introduces the risk of infection.
Whatever the
motivation, the company has done all it can to limit the use of
bevacizumab outside cancer, most notably by not applying for regulatory
approval for use in patients with macular degeneration. Some health
systems are finding ways round this, as Campbell and colleagues explain.
But the combination of legal threats, safety concerns, and financial
incentives to use ranibuzimab has maintained the more expensive drug’s
lucrative market. Sales in the US in 2010 topped $1.8bn.
In the UK, as Ingrid Torjesen reports (doi:10.1136/bmj.e3012),
efforts are under way to get bevacizumab approved for use in macular
degeneration despite resistance from Novartis. The National Institute
for Health and Clinical Excellence has said it could appraise the drug
if asked to by the Department of Health. Campbell and colleagues report
that the department is waiting for the results of the IVAN trial in the
UK, due to be published this month. But it is unlikely to resolve the
safety concerns. Neither this nor the CATT trial was big enough to
detect small but clinically relevant differences in adverse outcomes
such as stroke, they say. Long term postmarketing surveillance is needed
for that.
So what’s to be done in the best interests of
patients and the public purse? Campbell and colleagues call for clear
guidance to use bevacizumab from professional organisations, a review of
policies that discourage off-label use if there is good evidence for a
drug’s use, and better communication among health technology assessors
in different parts of the world.
Notes
Cite this as: BMJ 2012;344:e3162
Footnotes
- Follow BMJ Editor Fiona Godlee on Twitter @fgodlee and the BMJ @bmj_latest
Saturday, February 2, 2013
MYRISTOYLATION, A TRUE COMMITMENT AT THE CELLULAR MEMBRANE!
Proliferation, growth and spread of cancers is mainly driven by phenomena occurring at the membrane. A Molecular structure called SRC or sarc (for sarcoma) is anchored there, and Myristoylation is the process that keep it there. SARC or SRC
Wikipedia suggests:
Proto-oncogene tyrosine-protein kinase Src is an enzyme that in humans is encoded by the SRC gene.[1]
Src (pronounced "sarc" as it is short for sarcoma) is a proto-oncogene encoding a tyrosine kinase originally discovered by J. Michael Bishop and Harold E. Varmus, for which they were awarded the 1989 Nobel Prize in Physiology or Medicine.[2] It belongs to a family of non-receptor tyrosine kinases called Src family kinases. The discovery of Src family proteins has been instrumental to the modern understanding of cancer as a disease where normally healthy cellular signalling has gone awry.
This gene is similar to the v-src gene of Rous sarcoma virus. This proto-oncogene may play a role in the regulation of embryonic development and cell growth. The protein encoded by this gene is a tyrosine-protein kinase whose activity can be inhibited by phosphorylation by c-SRC kinase. Mutations in this gene could be involved in the malignant progression of colon cancer. Two transcript variants encoding the same protein have been found for this gene.[3]
==============================================================
In fact, SRC gene is at the center of Metastatic process through interaction with cell adhesion molecules, growth factors and many signal transduction pathways:
Wikipedia:
Interactions
Src (gene) has been shown to interact with
MET ACTIVITY INDUCED BY SRC ACTIVATION BY PHOSPHORYLATION SEEMS TO BE DETERMINED BY THE TYPE OF GROWTH FACTOR INDUCED. THROUGH HGFR, IT LEADS TO EMBRYONIC DEVELOPMENT IN THE EARLY CELL, AND TO WOUND HEALING IN RELEVANT TISSUE (SKIN), AND THROUGH HGF, IT LEADS TO MESENCHYMAL DIFFERENTIATION.
THROUGH MET, SRC LEADS TO RAS, PI3K/MAP K AND STAT EFFECTS
PHENOMENA AT THE MEMBRANE, A TRUE HISTORY, STILL UNFOLDING.
PLEASE ALSO NOTE CONFORMATION RAPPROCHEMENT BETWEEN SRC AND THE C-ABL WHERE IMITIMAB /GLEEVEC ACTS! SRC, CENTER OF METASTASIS, IS AN INTRODUCTION TO OUR FUTURE DISCUSSIONS.
Wikipedia suggests:
Src (gene)
From Wikipedia, the free encyclopedia
Jump to: navigation, search
| This article needs attention from an expert on the subject. Please add a reason or a talk parameter to this template to explain the issue with the article. Consider associating this request with a WikiProject. (March 2011) |
| V-src sarcoma (Schmidt-Ruppin A-2) viral oncogene homolog (avian) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
 PDB rendering based on 1a07. |
|||||||||||
|
|||||||||||
| Identifiers | |||||||||||
| Symbols | SRC; ASV; SRC1; c-SRC; p60-Src | ||||||||||
| External IDs | OMIM: 190090 MGI: 98397 HomoloGene: 21120 ChEMBL: 267 GeneCards: SRC Gene | ||||||||||
| EC number | 2.7.10.2 | ||||||||||
|
|||||||||||
| RNA expression pattern | |||||||||||
 |
|||||||||||
 |
|||||||||||
| More reference expression data | |||||||||||
| Orthologs | |||||||||||
| Species | Human | Mouse | |||||||||
| Entrez | 6714 | 20779 | |||||||||
| Ensembl | ENSG00000197122 | ENSMUSG00000027646 | |||||||||
| UniProt | P12931 | P05480 | |||||||||
| RefSeq (mRNA) | NM_005417.3 | NM_001025395.2 | |||||||||
| RefSeq (protein) | NP_005408.1 | NP_001020566.1 | |||||||||
| Location (UCSC) | Chr 20: 35.97 – 36.03 Mb |
Chr 2: 157.42 – 157.47 Mb |
|||||||||
| PubMed search | [1] | [2 | |||||||||
Src (pronounced "sarc" as it is short for sarcoma) is a proto-oncogene encoding a tyrosine kinase originally discovered by J. Michael Bishop and Harold E. Varmus, for which they were awarded the 1989 Nobel Prize in Physiology or Medicine.[2] It belongs to a family of non-receptor tyrosine kinases called Src family kinases. The discovery of Src family proteins has been instrumental to the modern understanding of cancer as a disease where normally healthy cellular signalling has gone awry.
This gene is similar to the v-src gene of Rous sarcoma virus. This proto-oncogene may play a role in the regulation of embryonic development and cell growth. The protein encoded by this gene is a tyrosine-protein kinase whose activity can be inhibited by phosphorylation by c-SRC kinase. Mutations in this gene could be involved in the malignant progression of colon cancer. Two transcript variants encoding the same protein have been found for this gene.[3]
==============================================================
In fact, SRC gene is at the center of Metastatic process through interaction with cell adhesion molecules, growth factors and many signal transduction pathways:
Wikipedia:
Interactions
Src (gene) has been shown to interact with
- AR,[19][20][21]
- ARNT,[22]
- AHR,[22]
- ADRBK1,[23]
- ADRB3,[24]
- BCAR1,[25][26][27][28][29][30]
- CD44,[31]
- DAB2,[32]
- DDEF1,[33]
- DAG1,[34]
- EPHB2,[35][36]
- EGFR,[37][38][39]
- EPS8,[40]
- ESR1,[19][41][42][43]
- ESR2,[19][43]
- GNB2L1,[44]
- GRB2,[23][45]
- GRIN2A,[46][47]
- HNF1A,[48]
- KHDRBS1,[49][50][51][52][53]
- MT-ND2,[54]
- MUC1,[55][56]
- NCOA6,[57][58][59][60]
- PDE6G,[23]
- PLD2,[61]
- PRKCZ,[62]
- PTK2,[26][30][63][64][65][66]
- PTK2B,[38][67][68]
- RAF1,[69]
- RASA1,[70][71]
- RARA,[42][72]
- RICS,[73]
- SRF,[74]
- SHB,[75]
- STAT1,[37][76]
- STAT3,[77] and
- WAS.[78][79]
See also
Genatlas:- c-SRC is non receptor tyrosine kinase, direct effector of G proteins
- c-SRC plays a role in the regulation of embryonic development and cell growth
- c-SRC has an appreciable role in the organization of the Golgi apparatus, which may be linked to its involvement in protein transport from the Golgi apparatus to the endoplasmic reticulum
- c-SRC has an appreciable role in the organization of the Golgi apparatus, which may be linked to its involvement in protein transport from the Golgi apparatus to the endoplasmic reticulum
- monopalmitoylated SFK required for VEGF mitogenic signaling with SRC and FYN, but maintaining distinct properties in the regulation of VEGF-mediated endothelial cell events (Pubmed 16400523)
- c-SRC regulates Golgi structure and KDELR1-dependent retrograde transport to the endoplasmic reticulum
- c-SRC has a key role in the maintenance of epithelial integrity (Pubmed 18305002)
- c-SRC is crucially involved in the ghrelin-mediated Akt activation (Pubmed 19262695)
- non palmitoylated SFK (Src-family tyrosine kinase), rapidly exchanged between the plasma membrane and late endosomes/lysosomes (suggest that SFK trafficking is specified by the palmitoylation state in the SH4 domain) (Pubmed 19258394)
- major kinase implicated in PTK2 phosphorylation, and is directly translocated from focal adhesions to membrane ruffles, thereby promoting formation of new adhesion complexes (Pubmed 19066724)
- interacting with PDLIM4 and PTPN13 (PDLIM4 suppresses SRC activation through interacting with SRC and PTPN13, allowing PTPN13-dependent dephosphorylation of SRC at the activation loop) (Pubmed 19307596)
- c-SRC inhibits SGK1-mediated phosphorylation hereby restoring the WNK4-mediated inhibition of ROMK channels thus suppressing K secretion) (Pubmed 19706464)
- c-SRC binds DVL2, a key phosphoprotein in Wnt signaling, at two positions: an SH3-binding domain and a C-terminal domain (Pubmed 19920076)
- ----------------------------------------------------------------------------------------------
- THIS SRC IS PIVOTAL AND CENTER TO ALL ACTIVITY INVOLVING THE SPREAD AND MAINTENANCE OF CANCER. IT IS SOMEWHAT CELL AND SPECIES SPECIFIC.
- WE PURPOSELY LEFT THE DASATINIB REFERENCE HERE TO INDICATE THAT SCIENTIST ARE TRYING TO TARGET THIS CROSS ROAD MOLECULAR STRUCTURE.
- IT'S ACTIVITY AT THE MET PROTEIN INSURE CANCER GROWTH AND VASCULARIZATION THROUGH MET EFFECT ON ANGIOGENESIS.
- REMEMBER MET DISTURBANCES ARE PROMINENT IN PAPILLARY RENAL CELL CANCER.
MET ACTIVITY INDUCED BY SRC ACTIVATION BY PHOSPHORYLATION SEEMS TO BE DETERMINED BY THE TYPE OF GROWTH FACTOR INDUCED. THROUGH HGFR, IT LEADS TO EMBRYONIC DEVELOPMENT IN THE EARLY CELL, AND TO WOUND HEALING IN RELEVANT TISSUE (SKIN), AND THROUGH HGF, IT LEADS TO MESENCHYMAL DIFFERENTIATION.
THROUGH MET, SRC LEADS TO RAS, PI3K/MAP K AND STAT EFFECTS
PHENOMENA AT THE MEMBRANE, A TRUE HISTORY, STILL UNFOLDING.
PLEASE ALSO NOTE CONFORMATION RAPPROCHEMENT BETWEEN SRC AND THE C-ABL WHERE IMITIMAB /GLEEVEC ACTS! SRC, CENTER OF METASTASIS, IS AN INTRODUCTION TO OUR FUTURE DISCUSSIONS.
By Eva Kiesler, PhD, Science Writer/Editor | Wednesday, December 19, 2012
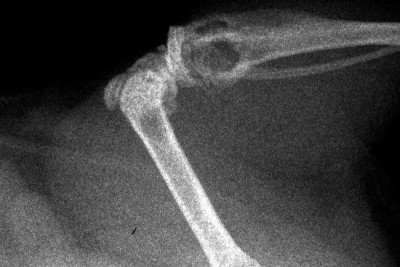 This X-ray image shows metastatic bone
tumors in a mouse with kidney cancer. The metastatic tumors appear as
hollow or dark areas in the bones.
This X-ray image shows metastatic bone
tumors in a mouse with kidney cancer. The metastatic tumors appear as
hollow or dark areas in the bones.
Metastasis, the process by
which some tumors spread from their site of origin to other body parts,
accounts for more than nine out of ten cancer-related deaths. But
scientists still know relatively little about the genes and biological
processes that cause metastasis to occur — information that potentially
could translate into new ways to stop cancers from reaching advanced or
terminal stages.
Now a Memorial Sloan-Kettering research team has shed light on the mechanisms by which kidney cancer metastasizes to distant organs, including the lungs, bone, and brain. Published in the journal Nature Medicine in December, the findings point to potential therapeutic strategies to control the spread of the disease. The study also offers scientific insights that could advance metastasis research in other cancer types.
“In this study, we focused on clear cell renal cell carcinoma, the most common subtype of kidney cancer, for which there is an urgent need for more-effective therapies,” says postdoctoral fellow Sakari Vanharanta, the first author of the Nature Medicine report. “The metastatic form of this disease is almost always incurable.”
In the vast majority of patients, clear cell renal cell carcinoma (ccRCC) tumors carry DNA changes in a gene called VHL. These mutations have been shown to cause the formation of primary kidney tumors, but they do not necessarily lead to metastasis. Until recently, researchers did not know what makes some renal cell carcinoma cells capable of forming secondary tumors in distant organs.
They discovered that two genes called CYTIP and CXCR4 are activated in metastatic tumor cells but inactive in non-metastatic cells. Their experiments suggest that the activation of the two genes might be essential for the spread of kidney cancer.
“CXCR4 has been linked to metastasis before in this and other tumor types, including breast cancer,” Dr. Vanharanta says. “Now, our study shows that blocking CXCR4 function with a drug called plerixafor can reduce kidney cancer metastasis in mice.” Plerixafor (MozobilTM) is currently used to stimulate blood stem cells in some cancer patients treated by bone marrow transplantation.
The researchers plan to investigate further whether CXCR4 and CYTIP, and other genes identified in the study, might offer new targets for the development of more-effective drugs for kidney cancer.
Unlike gene mutations, which alter a cell’s genetic code, epigenetic changes leave the DNA sequence unaffected. Nevertheless, such changes can influence a cell’s behavior by switching individual genes on or off.
Epigenetic modifications are commonly seen in many types of cancer and have recently been associated with more-advanced disease. However, little is known about the specific genes and mechanisms by which tumor cells may reconfigure their epigenetic makeup, causing a person’s disease to progress and establish itself in new organs.
“Our study has demonstrated with clear examples how epigenetic alterations can lead to the activation of metastasis-inducing genes,” Dr. Vanharanta notes. “This is a conceptual advancement that is likely to help us understand how metastasis occurs in kidney cancer as well as in other cancer types.”
Now a Memorial Sloan-Kettering research team has shed light on the mechanisms by which kidney cancer metastasizes to distant organs, including the lungs, bone, and brain. Published in the journal Nature Medicine in December, the findings point to potential therapeutic strategies to control the spread of the disease. The study also offers scientific insights that could advance metastasis research in other cancer types.
Focusing on Kidney Cancer
The research was led by cancer biologist Joan Massagué, Chair of the Sloan-Kettering Institute’s Cancer Biology and Genetics Program and Director of the Metastasis Research Center. In recent years, his laboratory has uncovered basic causes of metastasis in a number of cancer types, including breast and lung cancers.“In this study, we focused on clear cell renal cell carcinoma, the most common subtype of kidney cancer, for which there is an urgent need for more-effective therapies,” says postdoctoral fellow Sakari Vanharanta, the first author of the Nature Medicine report. “The metastatic form of this disease is almost always incurable.”
In the vast majority of patients, clear cell renal cell carcinoma (ccRCC) tumors carry DNA changes in a gene called VHL. These mutations have been shown to cause the formation of primary kidney tumors, but they do not necessarily lead to metastasis. Until recently, researchers did not know what makes some renal cell carcinoma cells capable of forming secondary tumors in distant organs.
New Potential Drug Targets
In the recent study, the team addressed this question by performing experiments in mouse models and cell lines, and by analyzing biological and clinical data from more than 700 patients with ccRCC, whose tumors had been analyzed in large-scale cancer genomics projects.They discovered that two genes called CYTIP and CXCR4 are activated in metastatic tumor cells but inactive in non-metastatic cells. Their experiments suggest that the activation of the two genes might be essential for the spread of kidney cancer.
“CXCR4 has been linked to metastasis before in this and other tumor types, including breast cancer,” Dr. Vanharanta says. “Now, our study shows that blocking CXCR4 function with a drug called plerixafor can reduce kidney cancer metastasis in mice.” Plerixafor (MozobilTM) is currently used to stimulate blood stem cells in some cancer patients treated by bone marrow transplantation.
The researchers plan to investigate further whether CXCR4 and CYTIP, and other genes identified in the study, might offer new targets for the development of more-effective drugs for kidney cancer.
Exploring the Epigenetics of Metastasis
In addition, the investigators explored the mechanisms by which the CXCR4 and CYTIP genes are switched on in kidney cancer cells to incite metastasis. Their study revealed that the genes undergo a series of epigenetic changes — modifications in the proteins that package a cell’s DNA and regulate genes.Unlike gene mutations, which alter a cell’s genetic code, epigenetic changes leave the DNA sequence unaffected. Nevertheless, such changes can influence a cell’s behavior by switching individual genes on or off.
Epigenetic modifications are commonly seen in many types of cancer and have recently been associated with more-advanced disease. However, little is known about the specific genes and mechanisms by which tumor cells may reconfigure their epigenetic makeup, causing a person’s disease to progress and establish itself in new organs.
“Our study has demonstrated with clear examples how epigenetic alterations can lead to the activation of metastasis-inducing genes,” Dr. Vanharanta notes. “This is a conceptual advancement that is likely to help us understand how metastasis occurs in kidney cancer as well as in other cancer types.”
Comments
Submitted by Hannu Vanharanta | Wednesday, December 19, 2012 - 11:00 AM.
Congratulations!!!! to Director Joan Massague and PostDoc Sakari
Vanharanta. This text was more understandable for me. Thank You!
Submitted by Jussi Kantola | Wednesday, December 19, 2012 - 1:55 PM.
Super research!! Congrats much!
Submitted by Tiina Malinen-Woodward | Friday, December 28, 2012 - 2:45 AM.
This is what we patients truly need: new approaches, brave research,
quick human tests! Thank you for your brilliant work! We live in
hope.
Submitted by Rohinton (Billy) Billimoria | Thursday, January 31, 2013 - 6:02 PM.
Congratulations to Director Joan Massague and PostDoc Sakari
Vanharanta!! I am a survivor of Renal Cell Carcinoma, which had
matastised in my lung, aorta and liver through a clinical trial
conducted by Dr. Motzer in 2004-2005 at MSKCC. It has been since then
that I have been cured and survived through the clinical trial that gave
me Pegalated Interferon. I am also a donor to MSKCC and am glad to see
the research projects providing great results. Keep it up, you guys out
there can do it and we can soon win the battles against cancer. God
Bless!
Submitted by Sandip Kumar Bajoria | Friday, February 1, 2013 - 12:42 AM.
Congrats to Drs on this valuable research. I am suffering from mRcc
with lungs and bones having metastasis. I hope new medicine will come
before I die. Sutent and Afinator have failed on me. Any valued advice
for my survival will be appreciated. I have also shown to Dr James Heish
of Sloan Memorial.
Submitted by Gautam Sakya | Friday, February 1, 2013 - 5:11 AM.
You have successfully blocked the gene CXCR4 in your laboratory mice
to prevent metastasis. I have removed the kidney with ccRCC, stage III
pT3a four and half years ago and no clear evidence of metastasis has
been found as yet in 6 monthly follow up CT scans. I wish, my RCC is the
same type as that of the mice and I would be glad to become a mice for a
limited period of time !
Add a Comment
Tagged in This Post
Related Cancer & Treatment Information
Related Topics
for further info go to original article!
CPRIT FAILURES
What exactly came out of the CPRIT experience so far and, we said this before, IT IS
lack of Vision, baldness and authenticity. It was conformity to existing systems and some greed!
CPRIT was taken over by University fund raising people who spent more time imagining ways to funnel money to Universities without asking those Universities to deliver something CPRIT should have wanted. Without definition of what was wanted by CPRIT, without a vision from CPRIT, there is no way to calibrate and measure University output. Limiting the number of research applications by university should not have become the only control mechanism of waste at CPRIT. How these research topics are relevant to the master plan at CPRIT and how much they will advance us toward cancer cure should have been the compass! How they fit into the plan and vision at CPRIT. It was shocking to me to hear the scientific leader at CPRIT saying she will not sit in meetings where funding will actually be attributed to avoid the ongoing fight among universities. Who is watching the plan for cancer cure if any at CPRIT! Once one has gotten the power it is time to shine by baldness and resistance to local undue pressures. Rise above politics, do not bend to pressure because it may skew your outcome. And clearly only the truth wins. Carry yourself true to the master plan because it is your only true friend and Compass. Religious people believe in the truth of the Gospel and not in the cloth and power of the pastor. And a Pastor is good when his words stick to the bible. Many pastors have been saved from politics within the church because the community support them, the community believe they speak the truth of the bible!
Those of us who have worked in mass implementation of public health program recognize the power of ANIMATORS in the adoption process of healthy behaviors. Dear ABBY is an example. Prevention programs need a GURU or two in Texas. Allowing Survivorship programs to be disseminated in 4 or 5 locations throughout the state to acquire and develop such persona is critical. People will not comply until they are convinced by messages from their leader/GURU. Not everybody of course listen to a guru in a free society but those who will, represent a gain for our society as long as the message is improving health behavior. 2 months ago, CPRIT was still looking at how success will look like.
When it comes to search for a cure, it is an open race. To send 42% of funds to one institution has no justification whatsoever. It is a demonstration of being under the thumb and nothing else. There is a miss opportunity here of grave consequences. CPRIT should do like most big organization, regional offices with real power to give fund based on local needs and local constraints. This the best way to address the variety of population needs in Texas, and limit disparity variation in assistance to communities. This concentration of power in Houston or Austin is not warranted and increase to susceptibility to political influence. Regional Komen foundation offices have dominion over local programs. Check with them! Their central office still control what is going on. But this way over 60% of tax money will not end up in one city (HOUSTON) as it is the case now at CPRIT.
let's give to CPRIT a better chance, a state dimension, people needs to know what is CPRIT. When we speak to our colleagues about CPRIT, many oncologists look at us as if we dropped from the moon. Only a handful of doctors have heard of it! CPRIT is a secret known only to few researchers. And Universities want to keep it that way. Some offices are now coming to CRBCM to train on how to best access CPRIT portal for future applications, Come on guys, 3 billions and you don't want people to know about! Even Medscape wrote a piece about CPRIT only after the CPRIT debacle happened!
who has the vision for the cure?
who has the baldness to create the new state wide CPRIT?
who will resist the power of the networking?
who wants all the people of Texas to get involved?
THAT IS THE LEADER NEEDED AT CPRIT!
What exactly came out of the CPRIT experience so far and, we said this before, IT IS
lack of Vision, baldness and authenticity. It was conformity to existing systems and some greed!
CPRIT was taken over by University fund raising people who spent more time imagining ways to funnel money to Universities without asking those Universities to deliver something CPRIT should have wanted. Without definition of what was wanted by CPRIT, without a vision from CPRIT, there is no way to calibrate and measure University output. Limiting the number of research applications by university should not have become the only control mechanism of waste at CPRIT. How these research topics are relevant to the master plan at CPRIT and how much they will advance us toward cancer cure should have been the compass! How they fit into the plan and vision at CPRIT. It was shocking to me to hear the scientific leader at CPRIT saying she will not sit in meetings where funding will actually be attributed to avoid the ongoing fight among universities. Who is watching the plan for cancer cure if any at CPRIT! Once one has gotten the power it is time to shine by baldness and resistance to local undue pressures. Rise above politics, do not bend to pressure because it may skew your outcome. And clearly only the truth wins. Carry yourself true to the master plan because it is your only true friend and Compass. Religious people believe in the truth of the Gospel and not in the cloth and power of the pastor. And a Pastor is good when his words stick to the bible. Many pastors have been saved from politics within the church because the community support them, the community believe they speak the truth of the bible!
Those of us who have worked in mass implementation of public health program recognize the power of ANIMATORS in the adoption process of healthy behaviors. Dear ABBY is an example. Prevention programs need a GURU or two in Texas. Allowing Survivorship programs to be disseminated in 4 or 5 locations throughout the state to acquire and develop such persona is critical. People will not comply until they are convinced by messages from their leader/GURU. Not everybody of course listen to a guru in a free society but those who will, represent a gain for our society as long as the message is improving health behavior. 2 months ago, CPRIT was still looking at how success will look like.
When it comes to search for a cure, it is an open race. To send 42% of funds to one institution has no justification whatsoever. It is a demonstration of being under the thumb and nothing else. There is a miss opportunity here of grave consequences. CPRIT should do like most big organization, regional offices with real power to give fund based on local needs and local constraints. This the best way to address the variety of population needs in Texas, and limit disparity variation in assistance to communities. This concentration of power in Houston or Austin is not warranted and increase to susceptibility to political influence. Regional Komen foundation offices have dominion over local programs. Check with them! Their central office still control what is going on. But this way over 60% of tax money will not end up in one city (HOUSTON) as it is the case now at CPRIT.
let's give to CPRIT a better chance, a state dimension, people needs to know what is CPRIT. When we speak to our colleagues about CPRIT, many oncologists look at us as if we dropped from the moon. Only a handful of doctors have heard of it! CPRIT is a secret known only to few researchers. And Universities want to keep it that way. Some offices are now coming to CRBCM to train on how to best access CPRIT portal for future applications, Come on guys, 3 billions and you don't want people to know about! Even Medscape wrote a piece about CPRIT only after the CPRIT debacle happened!
who has the vision for the cure?
who has the baldness to create the new state wide CPRIT?
who will resist the power of the networking?
who wants all the people of Texas to get involved?
THAT IS THE LEADER NEEDED AT CPRIT!
DIFFERENTIATION AND CANCER CURE II
Since our primary objective is to find "natural ways" for the cure. How does differentiation fit into the strategy. We have described how Differentiation is linked to suppression or silencing of certain genes (WT1), and over-expression of other (specific transcription factors or types of RAS). The cell under differentiation shows us how to do it to control gene expression (various pattern of Methylation). Even the location of the differentiation Vs another location where this differentiation does not occur, gives us local environmental conditions prohibiting such differentiation (why wings on the back and not the front). What environment makes the cell tick is important in Hematologic cancers.
The other opportunity that differentiation offers is that it bring natural cell death to even the cancer cell. Indeed full differentiation renders the cell susceptible to turn over mechanisms which includes Apoptosis.
The notion that amount of methylation is important for cell survival bring an interesting notion that we should be quantifying this parameter and registering pattern to differentiate cancer cells from normal cell. and that selective demethylation could be curative.
When you block differentiation, specific tissue development may be compromised all together. In such formed tissue, could blocking these tissue differentiation molecules affect more proliferating cells? Certain differentiation (Mucin production) are protective for the cancer (TIM-3). Silencing or antibody receptor to related growth factor allow for a weaker cancer. Can we switch differentiation of cancer cells into easier cancer to treat through manipulation of growth factor or alteration of splicing sequence or quantitative or qualitative change of transcription factors. By gene Knock out or silencing, can we either commit cells to full differentiation or remove cells ability to go back to a proliferative state....
Genes of Differentiation
WT 1
OCT4
SOX 4
KLF4
MYC
NANOG
PRC2
WINT
STAT3
NGF
Variable genes
RAS
CK5,6
YB-1
Nomenclature comment to follow
Since our primary objective is to find "natural ways" for the cure. How does differentiation fit into the strategy. We have described how Differentiation is linked to suppression or silencing of certain genes (WT1), and over-expression of other (specific transcription factors or types of RAS). The cell under differentiation shows us how to do it to control gene expression (various pattern of Methylation). Even the location of the differentiation Vs another location where this differentiation does not occur, gives us local environmental conditions prohibiting such differentiation (why wings on the back and not the front). What environment makes the cell tick is important in Hematologic cancers.
The other opportunity that differentiation offers is that it bring natural cell death to even the cancer cell. Indeed full differentiation renders the cell susceptible to turn over mechanisms which includes Apoptosis.
The notion that amount of methylation is important for cell survival bring an interesting notion that we should be quantifying this parameter and registering pattern to differentiate cancer cells from normal cell. and that selective demethylation could be curative.
When you block differentiation, specific tissue development may be compromised all together. In such formed tissue, could blocking these tissue differentiation molecules affect more proliferating cells? Certain differentiation (Mucin production) are protective for the cancer (TIM-3). Silencing or antibody receptor to related growth factor allow for a weaker cancer. Can we switch differentiation of cancer cells into easier cancer to treat through manipulation of growth factor or alteration of splicing sequence or quantitative or qualitative change of transcription factors. By gene Knock out or silencing, can we either commit cells to full differentiation or remove cells ability to go back to a proliferative state....
Genes of Differentiation
WT 1
OCT4
SOX 4
KLF4
MYC
NANOG
PRC2
WINT
STAT3
NGF
Variable genes
RAS
CK5,6
YB-1
Nomenclature comment to follow
Friday, February 1, 2013
Mayo Study: Common Diabetes Drug May Treat Ovarian Cancer
Sunday, December 02, 2012
Diabetic patients with ovarian cancer who took the drug metformin for their diabetes had a better survival rate than patients who did not take it, a study headed by Mayo Clinic shows. The findings, published early online in the journal Cancer, may play an important role for researchers as they study the use of existing medications to treat different or new diseases.Metformin is a widely prescribed drug to treat diabetes, and previous research by others has shown its promise for other cancers. The Mayo-led study adds ovarian cancer to the list.
Researchers compared the survival of 61 patients with ovarian cancer taking metformin and 178 patients who were not taking metformin. Sixty-seven percent of the patients who took metformin were surviving after five years, compared with 47 percent of those who did not take the medication. When the researchers analyzed factors such as the patients' body mass index, the severity of the cancer, type of chemotherapy and quality of surgery, they found that patients taking metformin were nearly four times likelier to survive, compared with those not taking the medication.
"Our study demonstrated improved survival in women with ovarian cancer that were taking metformin," says co-author Sanjeev Kumar, M.B.B.S., a Mayo Clinic gynecologic oncology fellow. "The results are encouraging, but as with any retrospective study, many factors cannot be controlled for us to say if there is a direct cause and effect. Rather, this is further human evidence for a potential beneficial effect of a commonly used drug which is relatively safe in humans. These findings should provide impetus for prospective clinical trials in ovarian cancer."
The results may pave the way for using metformin in large-scale randomized trials in ovarian cancer, researchers say. Given the high mortality rate of ovarian cancer, researchers say there is a great need to develop new therapies for ovarian cancer. Metformin may potentially be one of these options.
Other study authors are Alexandra Meuter, M.D., of Ludwig-Maximilians University, Munich, Germany; Shailendra Giri, Ph.D., and Ramandeep Rattan, Ph.D., of Henry Ford Health System; Jeremy Chien, Ph.D., of the University of Kansas Medical Center; Prabin Thapa, M.S.; Carrie Langstraat, M.D.; William Cliby, M.D.; and Viji Shridhar, Ph.D. of Mayo Clinic.
This work was supported, in whole or in part, by Fred C. and Katherine B. Andersen Foundation grant, and CA123249 and P50 CA136393 National Institutes of Health grants.
###
About Mayo Clinic
Mayo Clinic is a nonprofit worldwide leader in medical care, research and education for people from all walks of life. For more information, visit MayoClinic.com or MayoClinic.org/news.Journalists can become a member of the Mayo Clinic News Network for the latest health, science and research news and access to video, audio, text and graphic elements that can be downloaded or embedded.
Media Contact
Kelley Luckstein
507-284-5005 (days)
507-284-2511 (evenings)
newsbureau@mayo.edu
507-284-5005 (days)
507-284-2511 (evenings)
newsbureau@mayo.edu
Subscribe to:
Comments (Atom)



