By Eva Kiesler, PhD, Science Writer/Editor | Wednesday, December 19, 2012
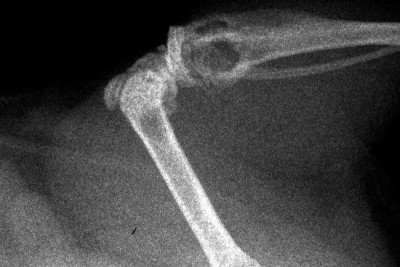 This X-ray image shows metastatic bone
tumors in a mouse with kidney cancer. The metastatic tumors appear as
hollow or dark areas in the bones.
This X-ray image shows metastatic bone
tumors in a mouse with kidney cancer. The metastatic tumors appear as
hollow or dark areas in the bones.
Metastasis, the process by
which some tumors spread from their site of origin to other body parts,
accounts for more than nine out of ten cancer-related deaths. But
scientists still know relatively little about the genes and biological
processes that cause metastasis to occur — information that potentially
could translate into new ways to stop cancers from reaching advanced or
terminal stages.
Now a Memorial Sloan-Kettering research team has shed light on the mechanisms by which kidney cancer metastasizes to distant organs, including the lungs, bone, and brain. Published in the journal Nature Medicine in December, the findings point to potential therapeutic strategies to control the spread of the disease. The study also offers scientific insights that could advance metastasis research in other cancer types.
“In this study, we focused on clear cell renal cell carcinoma, the most common subtype of kidney cancer, for which there is an urgent need for more-effective therapies,” says postdoctoral fellow Sakari Vanharanta, the first author of the Nature Medicine report. “The metastatic form of this disease is almost always incurable.”
In the vast majority of patients, clear cell renal cell carcinoma (ccRCC) tumors carry DNA changes in a gene called VHL. These mutations have been shown to cause the formation of primary kidney tumors, but they do not necessarily lead to metastasis. Until recently, researchers did not know what makes some renal cell carcinoma cells capable of forming secondary tumors in distant organs.
They discovered that two genes called CYTIP and CXCR4 are activated in metastatic tumor cells but inactive in non-metastatic cells. Their experiments suggest that the activation of the two genes might be essential for the spread of kidney cancer.
“CXCR4 has been linked to metastasis before in this and other tumor types, including breast cancer,” Dr. Vanharanta says. “Now, our study shows that blocking CXCR4 function with a drug called plerixafor can reduce kidney cancer metastasis in mice.” Plerixafor (MozobilTM) is currently used to stimulate blood stem cells in some cancer patients treated by bone marrow transplantation.
The researchers plan to investigate further whether CXCR4 and CYTIP, and other genes identified in the study, might offer new targets for the development of more-effective drugs for kidney cancer.
Unlike gene mutations, which alter a cell’s genetic code, epigenetic changes leave the DNA sequence unaffected. Nevertheless, such changes can influence a cell’s behavior by switching individual genes on or off.
Epigenetic modifications are commonly seen in many types of cancer and have recently been associated with more-advanced disease. However, little is known about the specific genes and mechanisms by which tumor cells may reconfigure their epigenetic makeup, causing a person’s disease to progress and establish itself in new organs.
“Our study has demonstrated with clear examples how epigenetic alterations can lead to the activation of metastasis-inducing genes,” Dr. Vanharanta notes. “This is a conceptual advancement that is likely to help us understand how metastasis occurs in kidney cancer as well as in other cancer types.”
Now a Memorial Sloan-Kettering research team has shed light on the mechanisms by which kidney cancer metastasizes to distant organs, including the lungs, bone, and brain. Published in the journal Nature Medicine in December, the findings point to potential therapeutic strategies to control the spread of the disease. The study also offers scientific insights that could advance metastasis research in other cancer types.
Focusing on Kidney Cancer
The research was led by cancer biologist Joan Massagué, Chair of the Sloan-Kettering Institute’s Cancer Biology and Genetics Program and Director of the Metastasis Research Center. In recent years, his laboratory has uncovered basic causes of metastasis in a number of cancer types, including breast and lung cancers.“In this study, we focused on clear cell renal cell carcinoma, the most common subtype of kidney cancer, for which there is an urgent need for more-effective therapies,” says postdoctoral fellow Sakari Vanharanta, the first author of the Nature Medicine report. “The metastatic form of this disease is almost always incurable.”
In the vast majority of patients, clear cell renal cell carcinoma (ccRCC) tumors carry DNA changes in a gene called VHL. These mutations have been shown to cause the formation of primary kidney tumors, but they do not necessarily lead to metastasis. Until recently, researchers did not know what makes some renal cell carcinoma cells capable of forming secondary tumors in distant organs.
New Potential Drug Targets
In the recent study, the team addressed this question by performing experiments in mouse models and cell lines, and by analyzing biological and clinical data from more than 700 patients with ccRCC, whose tumors had been analyzed in large-scale cancer genomics projects.They discovered that two genes called CYTIP and CXCR4 are activated in metastatic tumor cells but inactive in non-metastatic cells. Their experiments suggest that the activation of the two genes might be essential for the spread of kidney cancer.
“CXCR4 has been linked to metastasis before in this and other tumor types, including breast cancer,” Dr. Vanharanta says. “Now, our study shows that blocking CXCR4 function with a drug called plerixafor can reduce kidney cancer metastasis in mice.” Plerixafor (MozobilTM) is currently used to stimulate blood stem cells in some cancer patients treated by bone marrow transplantation.
The researchers plan to investigate further whether CXCR4 and CYTIP, and other genes identified in the study, might offer new targets for the development of more-effective drugs for kidney cancer.
Exploring the Epigenetics of Metastasis
In addition, the investigators explored the mechanisms by which the CXCR4 and CYTIP genes are switched on in kidney cancer cells to incite metastasis. Their study revealed that the genes undergo a series of epigenetic changes — modifications in the proteins that package a cell’s DNA and regulate genes.Unlike gene mutations, which alter a cell’s genetic code, epigenetic changes leave the DNA sequence unaffected. Nevertheless, such changes can influence a cell’s behavior by switching individual genes on or off.
Epigenetic modifications are commonly seen in many types of cancer and have recently been associated with more-advanced disease. However, little is known about the specific genes and mechanisms by which tumor cells may reconfigure their epigenetic makeup, causing a person’s disease to progress and establish itself in new organs.
“Our study has demonstrated with clear examples how epigenetic alterations can lead to the activation of metastasis-inducing genes,” Dr. Vanharanta notes. “This is a conceptual advancement that is likely to help us understand how metastasis occurs in kidney cancer as well as in other cancer types.”
Comments
Submitted by Hannu Vanharanta | Wednesday, December 19, 2012 - 11:00 AM.
Congratulations!!!! to Director Joan Massague and PostDoc Sakari
Vanharanta. This text was more understandable for me. Thank You!
Submitted by Jussi Kantola | Wednesday, December 19, 2012 - 1:55 PM.
Super research!! Congrats much!
Submitted by Tiina Malinen-Woodward | Friday, December 28, 2012 - 2:45 AM.
This is what we patients truly need: new approaches, brave research,
quick human tests! Thank you for your brilliant work! We live in
hope.
Submitted by Rohinton (Billy) Billimoria | Thursday, January 31, 2013 - 6:02 PM.
Congratulations to Director Joan Massague and PostDoc Sakari
Vanharanta!! I am a survivor of Renal Cell Carcinoma, which had
matastised in my lung, aorta and liver through a clinical trial
conducted by Dr. Motzer in 2004-2005 at MSKCC. It has been since then
that I have been cured and survived through the clinical trial that gave
me Pegalated Interferon. I am also a donor to MSKCC and am glad to see
the research projects providing great results. Keep it up, you guys out
there can do it and we can soon win the battles against cancer. God
Bless!
Submitted by Sandip Kumar Bajoria | Friday, February 1, 2013 - 12:42 AM.
Congrats to Drs on this valuable research. I am suffering from mRcc
with lungs and bones having metastasis. I hope new medicine will come
before I die. Sutent and Afinator have failed on me. Any valued advice
for my survival will be appreciated. I have also shown to Dr James Heish
of Sloan Memorial.
Submitted by Gautam Sakya | Friday, February 1, 2013 - 5:11 AM.
You have successfully blocked the gene CXCR4 in your laboratory mice
to prevent metastasis. I have removed the kidney with ccRCC, stage III
pT3a four and half years ago and no clear evidence of metastasis has
been found as yet in 6 monthly follow up CT scans. I wish, my RCC is the
same type as that of the mice and I would be glad to become a mice for a
limited period of time !


No comments:
Post a Comment