PTK2:
From Wikipedia, the free encyclopedia
| Protein tyrosine kinase 2 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
 PDB rendering of the C-terminal FAT domain based on 1k04[1]. |
|||||||||||
|
|||||||||||
| Identifiers | |||||||||||
| Symbols | PTK2; FADK; FAK; FAK1; FRNK; PPP1R71; p125FAK; pp125FAK | ||||||||||
| External IDs | OMIM: 600758 MGI: 95481 HomoloGene: 7314 ChEMBL: 2695 GeneCards: PTK2 Gene | ||||||||||
| EC number | 2.7.10.2 | ||||||||||
|
|||||||||||
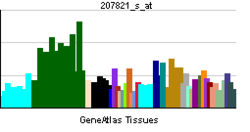
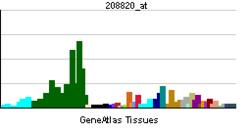
| RNA expression pattern | |||||||||||
 |
|||||||||||
 |
|||||||||||
| More reference expression data | |||||||||||
| Orthologs | |||||||||||
| Species | Human | Mouse | |||||||||
| Entrez | 5747 | 14083 | |||||||||
| Ensembl | ENSG00000169398 | ENSMUSG00000022607 | |||||||||
| UniProt | Q05397 | P34152 | |||||||||
| RefSeq (mRNA) | NM_001199649 | NM_001130409 | |||||||||
| RefSeq (protein) | NP_001186578 | NP_001123881 | |||||||||
| Location (UCSC) | Chr 8: 141.67 – 142.01 Mb |
Chr 15: 73.21 – 73.42 Mb |
|||||||||
| PubMed search | [1] | [2] | |||||||||
=============================================================================
AND THEY ARE PLENTY TALKED ABOUT!
=============================================================== I.E....
"Integrin-dependent translocation of phosphoinositide 3-kinase to the cytoskeleton of thrombin-activated platelets involves specific interactions of p85 alpha with actin filaments and focal adhesion kinase(JCB)"
The point is that at the membrane healing should occur after the "integrin" has been plucked off, but failure to heal may trigger the "cheloid effect". In the cell, this is where the Src gene is, the Wnt (catenins) and the Notch are here, Caspase 3 is present, and death Receptors,etc... (things can get complicated really fast with these guys around! unless of course phosphorylation or other taming mechanisms come to play!)
Focal Adhesion kinases (FAK)
". FAK is typically located at structures known as focal adhesions, these are multi-protein structures that link the extracellular matrix (ECM) to the cytoplasmic cytoskeleton. Additional components of focal adhesions include actin, filamin, vinculin, talin, paxillin, tensin[7] and RSU-1." This is what Taxol and Taxotere find their might! (components of microtubules)
remember tensin is same as PTEN
NIH
" PTEN1
- Also known as
- BZS; DEC; CWS1; GLM2; MHAM; TEP1; MMAC1; PTEN1; 10q23del
- Summary
- This gene was identified as a tumor suppressor that is mutated in a large number of cancers at high frequency. The protein encoded this gene is a phosphatidylinositol-3,4,5-trisphosphate 3-phosphatase. It contains a tensin like domain as well as a catalytic domain similar to that of the dual specificity protein tyrosine phosphatases. Unlike most of the protein tyrosine phosphatases, this protein preferentially dephosphorylates phosphoinositide substrates. It negatively regulates intracellular levels of phosphatidylinositol-3,4,5-trisphosphate in cells and functions as a tumor suppressor by negatively regulating AKT/PKB signaling pathway. [provided by RefSeq, Jul 2008]"