A blog about research, awareness, prevention, treatment and survivorship of Breast Cancer and all cancers, including targeted scientific research and a grassroots approach to increase screening for cancer, especially in the low income and under-insured population of El Paso, Texas, with a view to expand this new health care model to many other 'minority' populations across the United States and beyond
Showing posts with label cyclins. Show all posts
Showing posts with label cyclins. Show all posts
Monday, November 4, 2013
Digging deeper ! Chromatin remodeling
As we are progressing deeper into our understanding of disease pathophysiology, we are discovering that some of the cancers are caused or exacerbated by abnormalities of cyclin pathways (Triple negative breast cancers). There are disturbances at Cyclins and Hormone Receptors at the membranes. But deeper into the cells, there is disturbance at the Histones (epigenetic zone) where modulation is needed for transcriptions factors to be formed and unleashed. Here, the dance is governed by the PRBM1, BAFs, PBAF, BRDs, RSC, ARIDs and SWI/SNF (Chromatin remodeling). This is where BRAC-1 also plays its main function. Indeed some of the Adaptors or cofactor fail the BRCA-1 to continue to repair DNAs! And some cytokines fail here! we are working deeper!
Labels:
ARID,
BAF,
BRCA,
BRCA-1,
BRD,
chromatin remodeling,
crbcm,
cyclins,
hormone receptors,
kankonde,
PBAF,
PRBM1,
RSC,
SWI/SNF
Friday, August 23, 2013
THE "CHELOID FACTOR" AT THE CELLULAR MEMBRANE!
We tend to be excited about intracellular pathways as they travel through the Cytosol and affect epigenetic and nuclear phenomena. And our excitement has been justified since we have been able to affect cellular life by targeting various pathway molecules. But one should stress a particular event occurring at the membrane that mimics "wound phenomena". Aside for providing a physical boundary of the cell, the membrane is one of the most important "organs" of the cell. It is in itself a very chemically vibrant living "cellular tissue ". When you start reading about the cell they tell you about the layers of proteins and lipids that make up the cellular membranes. But this picture is far from the truth, the membrane is like the wall of a brick house. With each brick different from the next. Some of these bricks are called Integrins (I guess because they are an integral part of the membrane). Some of these bricks have a Cyclin, some have a growth factor! In fact, the membrane here serves as a reserve of these molecules. Some bricks can be divided in 2 portions. One portion that can "FLIP" inside when needed (This portion contains the cyclin, for example) and one portion that can "FLOP" outside (this portion contains a Metalloprotease). (see my post on FLIPPASE and FLOPPASE) The point is that once the brick is used there remains a hole with sharp edges. These edges are called "FOCAL ADHESION Molecules" (KINASES) in a cell and are governed by the PTK2 gene! (and of course PYK2)
PTK2 protein tyrosine kinase 2 (PTK2), also known as Focal Adhesion Kinase (FAK), is a protein that, in humans, is encoded by the PTK2 gene.[2] PTK2 is a focal adhesion-associated protein kinase
involved in cellular adhesion (how cells stick to each other and their
surroundings) and spreading processes (how cells move around).[3] It has been shown that when FAK was blocked, breast cancer cells became less metastastic due to decreased mobility.[4](Wikepedia
=============================================================================
AND THEY ARE PLENTY TALKED ABOUT!
=============================================================== I.E....
PTK2:
From Wikipedia, the free encyclopedia
Jump to: navigation, search
| Protein tyrosine kinase 2 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
 PDB rendering of the C-terminal FAT domain based on 1k04[1]. |
|||||||||||
|
|||||||||||
| Identifiers | |||||||||||
| Symbols | PTK2; FADK; FAK; FAK1; FRNK; PPP1R71; p125FAK; pp125FAK | ||||||||||
| External IDs | OMIM: 600758 MGI: 95481 HomoloGene: 7314 ChEMBL: 2695 GeneCards: PTK2 Gene | ||||||||||
| EC number | 2.7.10.2 | ||||||||||
|
|||||||||||
| RNA expression pattern | |||||||||||
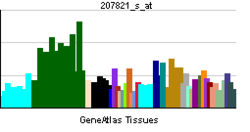 |
|||||||||||
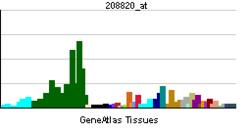 |
|||||||||||
| More reference expression data | |||||||||||
| Orthologs | |||||||||||
| Species | Human | Mouse | |||||||||
| Entrez | 5747 | 14083 | |||||||||
| Ensembl | ENSG00000169398 | ENSMUSG00000022607 | |||||||||
| UniProt | Q05397 | P34152 | |||||||||
| RefSeq (mRNA) | NM_001199649 | NM_001130409 | |||||||||
| RefSeq (protein) | NP_001186578 | NP_001123881 | |||||||||
| Location (UCSC) | Chr 8: 141.67 – 142.01 Mb |
Chr 15: 73.21 – 73.42 Mb |
|||||||||
| PubMed search | [1] | [2] | |||||||||
=============================================================================
AND THEY ARE PLENTY TALKED ABOUT!
=============================================================== I.E....
"Integrin-dependent translocation of phosphoinositide 3-kinase to the cytoskeleton of thrombin-activated platelets involves specific interactions of p85 alpha with actin filaments and focal adhesion kinase(JCB)"
The point is that at the membrane healing should occur after the "integrin" has been plucked off, but failure to heal may trigger the "cheloid effect". In the cell, this is where the Src gene is, the Wnt (catenins) and the Notch are here, Caspase 3 is present, and death Receptors,etc... (things can get complicated really fast with these guys around! unless of course phosphorylation or other taming mechanisms come to play!)
Focal Adhesion kinases (FAK)
". FAK is typically located at structures known as focal adhesions, these are multi-protein structures that link the extracellular matrix (ECM) to the cytoplasmic cytoskeleton. Additional components of focal adhesions include actin, filamin, vinculin, talin, paxillin, tensin[7] and RSU-1." This is what Taxol and Taxotere find their might! (components of microtubules)
remember tensin is same as PTEN
NIH
" PTEN1
- Also known as
- BZS; DEC; CWS1; GLM2; MHAM; TEP1; MMAC1; PTEN1; 10q23del
- Summary
- This gene was identified as a tumor suppressor that is mutated in a large number of cancers at high frequency. The protein encoded this gene is a phosphatidylinositol-3,4,5-trisphosphate 3-phosphatase. It contains a tensin like domain as well as a catalytic domain similar to that of the dual specificity protein tyrosine phosphatases. Unlike most of the protein tyrosine phosphatases, this protein preferentially dephosphorylates phosphoinositide substrates. It negatively regulates intracellular levels of phosphatidylinositol-3,4,5-trisphosphate in cells and functions as a tumor suppressor by negatively regulating AKT/PKB signaling pathway. [provided by RefSeq, Jul 2008]"
Friday, April 5, 2013
Nomenclature of 2 important genes in Ovarian cancer !
1.RASSF1A: One of the thing cancer cell do is to Methylate some genes in order to block its path to death.
it appears this gene is a critical door to shut or disable. It not only decrease the significance of RAS and MAPK in the pathogenesis of tumor that harbor this mutation. It also remove blockage to proliferation by desensitizing the cell to the effect of P53, Cyclins. Desensitize the cell to Death Receptor 6 and its Fas connection. RASSF1a, demethylation is a valid target in ovarian cancer.
2.HNF1B: " Hepatocyte Nuclear Factor 1α (HNF1α) is an atypical homeodomain-containing transcription factor that transactivates liver-specific genes including albumin, α-1-antitrypsin and α- and β-fibrinogen. Biallelic inactivating mutations of HNF1A have been frequently identified in hepatocellular adenomas (HCA), rare benign liver tumors usually developed in women under oral contraceptives, and in rare cases of hepatocellular carcinomas developed in non-cirrhotic liver. HNF1α-mutated HCA (H-HCA) are characterized by a marked steatosis and show activation of glycolysis, lipogenesis, translational machinery and mTOR pathway. We studied the consequences of HNF1α silencing in hepatic cell lines, HepG2 and Hep3B and we reproduced most of the deregulations identified in H-HCA."
(Laura Pelletier et al)
This gene is the gene of differentiation for liver formation, it has the structure of a CBF (core binding Factor) therefore has a subunit binding the DNA, therefore silencing that portion, and another subunit having locations for enzymatic proteins or molecular structures that directly assume various functions intended by the cell (formation of Albumin, alpha Antitrypsine, and Beta Fibrinogen).
Interestingly enough, Steatosis is a prominent feature here. This structure and gene may be of interest in LIPOSARCOMA?
DOES ACTIVATION OF MTOR DEMONSTRATED HERE OPEN THE DOOR TO THE USE OF MTOR IN LIPOSARCOMA?
it appears this gene is a critical door to shut or disable. It not only decrease the significance of RAS and MAPK in the pathogenesis of tumor that harbor this mutation. It also remove blockage to proliferation by desensitizing the cell to the effect of P53, Cyclins. Desensitize the cell to Death Receptor 6 and its Fas connection. RASSF1a, demethylation is a valid target in ovarian cancer.
2.HNF1B: " Hepatocyte Nuclear Factor 1α (HNF1α) is an atypical homeodomain-containing transcription factor that transactivates liver-specific genes including albumin, α-1-antitrypsin and α- and β-fibrinogen. Biallelic inactivating mutations of HNF1A have been frequently identified in hepatocellular adenomas (HCA), rare benign liver tumors usually developed in women under oral contraceptives, and in rare cases of hepatocellular carcinomas developed in non-cirrhotic liver. HNF1α-mutated HCA (H-HCA) are characterized by a marked steatosis and show activation of glycolysis, lipogenesis, translational machinery and mTOR pathway. We studied the consequences of HNF1α silencing in hepatic cell lines, HepG2 and Hep3B and we reproduced most of the deregulations identified in H-HCA."
(Laura Pelletier et al)
This gene is the gene of differentiation for liver formation, it has the structure of a CBF (core binding Factor) therefore has a subunit binding the DNA, therefore silencing that portion, and another subunit having locations for enzymatic proteins or molecular structures that directly assume various functions intended by the cell (formation of Albumin, alpha Antitrypsine, and Beta Fibrinogen).
Interestingly enough, Steatosis is a prominent feature here. This structure and gene may be of interest in LIPOSARCOMA?
DOES ACTIVATION OF MTOR DEMONSTRATED HERE OPEN THE DOOR TO THE USE OF MTOR IN LIPOSARCOMA?
Labels:
crrbcm,
cyclins,
demethylation,
hepatocellular carcinoma,
hepatocyte nuclera factor,
HNF1A,
HNF1B,
liposarcoma,
MAPK,
methylation,
mtor,
MTOR expression,
ovarian cancer,
RAS,
RASSF1A,
steatosis
Tuesday, March 19, 2013
Ovarian Cancer
*Researchers who conducted the study who conducted the use of Selumetinib in low grade serous Ovarian cancer were still puzzled because its activity did not follow presence of KRAS or BRAF. They have been wondering if it used another pathway. But remember MEK is the revolving door to de-differentiation and to the reversal of mesengialization and as such increase tor susceptibility not only to chemotherapy drugs, but also to the secondary angiogenic potentiation of MEK. That is, with anti-MEK, there is a down regulation of MAPK (as suggested) and therefore the C-JUN and TGF and cyclins, but also down regulation of the VEGF!
They say " Our results suggest that selumetinib is an active agent, but not necessarily because of BRAF or KRAS mutational activation per se,” the authors concluded.
They say " Our results suggest that selumetinib is an active agent, but not necessarily because of BRAF or KRAS mutational activation per se,” the authors concluded.
In an interview, Gershenson said that one reason for the
lack of correlation could be biomarker instability. Among the 52
patients, specimens were available for only 40, mutational analysis was
done in 34, and in 28 of those the tissue was from the primary therapy
and not from the recurrent tumor. “The question arises, are these
biomarkers stable over time or do they change, so that what you find in
the primary tumor may not be what you find in the recurrent tumor,” he
said." They suggesting here that the lack of correlation could be due to a changing nature of Biomarker. But the existence of other factors and pathways could not be be excluded!
Gene-Expression Profiling May Help Select Best Drugs for Pancreatic Cancer
Caroline Helwick
---------------------------------------------------------------------------------------------------
In this study patient with pancreatic cancer had reportedly circulating cell and their genes could predict response to therapy but unfortunately they did not spell out which genes were reviewed. We will investigate further this article...
Monday, March 11, 2013
THE CRCBM RECOMMENDS THIS PIECE OF ARTICLE TO ALL READERS!
Mechanisms of Resistance to Anti-Angiogenic Therapy and Development of Third-Generation Anti-Angiogenic Drug Candidates
+ Author Affiliations
- P. Carmeliet, MD, PhD, Vesalius Research Center, VIB, K.U. Leuven, Campus Gasthuisberg, Herestraat 49, B-3000, Leuven, Belgium Email: peter.carmeliet@med.kuleuven.be
- -----------------------------------------------------------------------------------------
- Suffice is to say that the concerns mentioned in this review, which is an excellent review, unveils in pretty good details the insufficiency of a monotherapy attacking an essential function of the cells. Not only will the cell have an answer such as dummy receptors, secondary amplification of transcription factors of growth factors, but escape mechanisms that include escape of the area leading to metastasis. I should confess that recruiting other cells to help fight the attacker (Myeloid and endothelial cells) showed clearly how much angiogenesis is globally needed. I would think that the reaction by the NF-kB would be sufficient; with its secondary growth factor production, induction would be the predictable way. But clearly, the cell wants restoration of the angiogenic function and finally wins, making Avastin effects short lived. By inducing Hypoxia, stress becomes a secondary impetus and c-JUN enters the dance and fights again with resulting amplification of growth factor and various dislocation of various cyclins at integrin locations including the Angiopoietins.
- One of the things that needs to be emphasized or not looked at or discussed in your piece are events happening at the MEK. You know by now that MEK is clearly amplified either by the cancerous process or in reaction to the blockage or consumption at VEGF. Tracking MEK is important, because if amplified and mutated it may reverse mesengial transformation and render the cell more omnipotent. It may be at the center of the observation that blocking both EGFR and VEGF reduces the progression free survival. Events at the MEK need to be scrutinized.
- You also realize that, in the long run, MTOR will be secondarily stimulated leading to Telomere preservation (stabilization) and cell surviva
- The quick restoration of the angiogenic function after cessation of the treatment marks the importance of VEGF.
Clearly, Avastin is never meant to be a monotherapy, that is the answer! To all action, there is a reaction. And cells expect action, it is built for them!
Monday, March 4, 2013
The AML discussion continues!
AML-1 mostly corresponds to the alpha subunit of the CBF and contains the RUNX-1 which specializes in hematologic differentiation of the cell, the other portion already contains among other things regulatory or catalytic molecules facilitating many processes of the cell combination with EVI-1 will control signal pathways and growth factors.
This type of AML, or AML in general, appears to be a disease mostly driven by dysregulation of promoters and regulator genes with global suppression of the NK-kB a nd the cyclins/TGF.
?role of ANDROGEN, interferon and growth factor in EVI-1 positive AML can be raised as a question. On top of standard induction chemotherapy of course! This is where Cyclosporine and Antithymocyte Globulin could still have a role (in EVI-1 AML)
One may wonder why TGF is suppressed? Maybe to stop the cancerous process to form a mass and stay "fluid"? That is, in granulocytic sarcoma, TGF would be less suppressed?
This type of AML, or AML in general, appears to be a disease mostly driven by dysregulation of promoters and regulator genes with global suppression of the NK-kB a nd the cyclins/TGF.
?role of ANDROGEN, interferon and growth factor in EVI-1 positive AML can be raised as a question. On top of standard induction chemotherapy of course! This is where Cyclosporine and Antithymocyte Globulin could still have a role (in EVI-1 AML)
One may wonder why TGF is suppressed? Maybe to stop the cancerous process to form a mass and stay "fluid"? That is, in granulocytic sarcoma, TGF would be less suppressed?
Saturday, February 16, 2013
HYPOTHESIS : WHERE DO CYCLINS COME FROM?
There is increasing evidence that Cyclins are integrins and so are Tumor growth factors, Tumor Necrosis factors, interleukins and interferons.
All these are membrane proteins with a particularity to be released from Metalloprotease and related adhesion molecules depending on the nature of stimuli. The discovery and description of ADAMs as type I membrane protein containing Metalloproteinase and an integrin domain locate the growth factors and Cyclins squarely at the membrane (surface and reticulum membrane). These proteins, once released, go straight to the Nucleus to unveil their might by activating transcription factor. In their track to the nucleus they can amplify and activate signal transduction pathways as well as either molecules. The cyclins find cytoplasmic and protein substrates (mostly enzymes) which have their specific domains and link to the site to activate them most of the time, changing their shapes so as to expose hidden electrons or atomic groups (such SH) to cause downstream chain activation.
Now as the pathway unfolds at light speed (or electronic speed) it may overwhelm the cell, protection has to be assured to hide death domains (which also are integrins and therefore at the membrane) and pathways to Apoptosis. Protection at the membrane seems to be offered by the INK while the CIP/Kip. But deep in the cell are the Bcl-like proteins. The CIP/Kip seems to work like Decoy specific proteins since the have Cyclin domain to stop them from stimulating their respective CDKs (Cyclin dependent Kinases). Some CDKs need 2 or more different stimulations to accomplish their deed. And with the number of stimulations comes the consequent activation of various substrates. The Retinoblastoma substrate governs the G1 progression phase in the cell cycle, but it needs at least 2 activations, first by Cyclin D followed by activation by Cyclin E in order for it to free E2F that light up tarnscrptions genes which control the path to S-phase. This Cyclin E also activates processes leading to Histone Biosynthesis, Centrosome activity and DNA replication. And in fact, Cyclin E is the one that leads to gene instability that characterize many triple negative breast cancers
(E2F AND CYCLIN E, ARE POWERFUL TARGETS FOR CANCER CURE)
One of the CIP/Kip(s) is the P21 which plays a role in the cell cycle arrest due to P53 activation.
I should note that the Kinase itself may be mutated. CDK4 is mutated in Melanoma, it renders the INK4 protein unable to occupy its domain and therefore is free to affect the nuclear transcription factor. Therefore the solution is to increase the ligand to INK4 so as to increase its ubiquitination and and degradation through the proteasome (Ipilimumab/CTLA 4 in T cell/ does not do this unfortunately, so there is more room for you to research). YES, LIKE FOR MERCEDES, WE NEED THE E CLASS OF PROTEINS TO FURTHER UBIQUITINATION. A MUTATION IN E CLASS (WHICH INCLUDES MDM2) WILL BE BAD IN MELANOMA!
Suffice is to show that what starts at the membrane moves quickly to the nucleus in a milli-milli second in a flash and turn the life of the host around!
It is worth noting that not only Cyclins can be blocked from entering the Nucleus where they trigger transcription factor formation, but sometimes the Decoy (Cip/Kip) is stopped from entering the nucleus and cannot stop Cyclins which have entered the nucleus: this happens in breast cancer (p27 mislocation)
-----------------------------------------------------------------------------------------
THE INTEGRINS, PRESUMED SOURCE OF CYCLINS!
There is increasing evidence that Cyclins are integrins and so are Tumor growth factors, Tumor Necrosis factors, interleukins and interferons.
All these are membrane proteins with a particularity to be released from Metalloprotease and related adhesion molecules depending on the nature of stimuli. The discovery and description of ADAMs as type I membrane protein containing Metalloproteinase and an integrin domain locate the growth factors and Cyclins squarely at the membrane (surface and reticulum membrane). These proteins, once released, go straight to the Nucleus to unveil their might by activating transcription factor. In their track to the nucleus they can amplify and activate signal transduction pathways as well as either molecules. The cyclins find cytoplasmic and protein substrates (mostly enzymes) which have their specific domains and link to the site to activate them most of the time, changing their shapes so as to expose hidden electrons or atomic groups (such SH) to cause downstream chain activation.
Now as the pathway unfolds at light speed (or electronic speed) it may overwhelm the cell, protection has to be assured to hide death domains (which also are integrins and therefore at the membrane) and pathways to Apoptosis. Protection at the membrane seems to be offered by the INK while the CIP/Kip. But deep in the cell are the Bcl-like proteins. The CIP/Kip seems to work like Decoy specific proteins since the have Cyclin domain to stop them from stimulating their respective CDKs (Cyclin dependent Kinases). Some CDKs need 2 or more different stimulations to accomplish their deed. And with the number of stimulations comes the consequent activation of various substrates. The Retinoblastoma substrate governs the G1 progression phase in the cell cycle, but it needs at least 2 activations, first by Cyclin D followed by activation by Cyclin E in order for it to free E2F that light up tarnscrptions genes which control the path to S-phase. This Cyclin E also activates processes leading to Histone Biosynthesis, Centrosome activity and DNA replication. And in fact, Cyclin E is the one that leads to gene instability that characterize many triple negative breast cancers
(E2F AND CYCLIN E, ARE POWERFUL TARGETS FOR CANCER CURE)
One of the CIP/Kip(s) is the P21 which plays a role in the cell cycle arrest due to P53 activation.
I should note that the Kinase itself may be mutated. CDK4 is mutated in Melanoma, it renders the INK4 protein unable to occupy its domain and therefore is free to affect the nuclear transcription factor. Therefore the solution is to increase the ligand to INK4 so as to increase its ubiquitination and and degradation through the proteasome (Ipilimumab/CTLA 4 in T cell/ does not do this unfortunately, so there is more room for you to research). YES, LIKE FOR MERCEDES, WE NEED THE E CLASS OF PROTEINS TO FURTHER UBIQUITINATION. A MUTATION IN E CLASS (WHICH INCLUDES MDM2) WILL BE BAD IN MELANOMA!
Suffice is to show that what starts at the membrane moves quickly to the nucleus in a milli-milli second in a flash and turn the life of the host around!
It is worth noting that not only Cyclins can be blocked from entering the Nucleus where they trigger transcription factor formation, but sometimes the Decoy (Cip/Kip) is stopped from entering the nucleus and cannot stop Cyclins which have entered the nucleus: this happens in breast cancer (p27 mislocation)
-----------------------------------------------------------------------------------------
THE INTEGRINS, PRESUMED SOURCE OF CYCLINS!
Saturday, December 29, 2012
AS WE SEARCH FOR THE CURE, IT IS IMPORTANT TO REMEMBER THAT:
Molecular Biology offers tremendous opportunities to fight cancer. In fact, it is surprising that we seem so early (or late, depending on how you understand this) in the game. The cell can be affected in so many ways that we are late reacting. Using integrated methods and our computer abilities, we should by now be involved in developing patterns of attacks by cancer cell type.
We should define clearly the major drivers by type of cancer, and pick the counter attacks specifically per type of cancer. We should be at the stage where each patient who presents to us has his genotype defined, changes in his membrane receptors described, driver mutations enunciated, status of P53, level of major Cyclins and various cell protections (P-gp, Bcl-2), status and quantitative expression of transcription factors, expression of Metastatic potential (E-Cadherin, Metallo-protease, TGF), histone conformation, level of Endonuclases, status of mitotic speed, types of protein Anchor at cell membranes, and Kinesins (Kif2a,b,c) and so on so forth, all spelled out on his file!
IT IS ONLY WITH THIS LEVEL OF DEFINITION, THAT WE CAN PICK AND CHOOSE AN APPROPRIATE TREATMENT, OR UNDERSTAND THE SHORTCOMINGS OF OUR CURRENT STANDARD TREATMENTS. Computers should also be used to tell us if combination treatments should be used sequentially or concurrently, and at which sequences, order and time our therapeutics should be given.
Molecular Biology, so many "distractions" and stuff that some scientists are spending days on, and may lead to something some day, but as we work in a race against death situation, and people are dying every day, it is time to pause and regroup, look at how to create this panel per patient, and develop computer supported patterns of therapy. And every 2-5 years make a stop, update our computer and reload for the Cure!
Molecular Biology offers tremendous opportunities to fight cancer. In fact, it is surprising that we seem so early (or late, depending on how you understand this) in the game. The cell can be affected in so many ways that we are late reacting. Using integrated methods and our computer abilities, we should by now be involved in developing patterns of attacks by cancer cell type.
We should define clearly the major drivers by type of cancer, and pick the counter attacks specifically per type of cancer. We should be at the stage where each patient who presents to us has his genotype defined, changes in his membrane receptors described, driver mutations enunciated, status of P53, level of major Cyclins and various cell protections (P-gp, Bcl-2), status and quantitative expression of transcription factors, expression of Metastatic potential (E-Cadherin, Metallo-protease, TGF), histone conformation, level of Endonuclases, status of mitotic speed, types of protein Anchor at cell membranes, and Kinesins (Kif2a,b,c) and so on so forth, all spelled out on his file!
IT IS ONLY WITH THIS LEVEL OF DEFINITION, THAT WE CAN PICK AND CHOOSE AN APPROPRIATE TREATMENT, OR UNDERSTAND THE SHORTCOMINGS OF OUR CURRENT STANDARD TREATMENTS. Computers should also be used to tell us if combination treatments should be used sequentially or concurrently, and at which sequences, order and time our therapeutics should be given.
Molecular Biology, so many "distractions" and stuff that some scientists are spending days on, and may lead to something some day, but as we work in a race against death situation, and people are dying every day, it is time to pause and regroup, look at how to create this panel per patient, and develop computer supported patterns of therapy. And every 2-5 years make a stop, update our computer and reload for the Cure!
Monday, December 17, 2012
STRATEGIES FOR THE CURE
Since the work of Weinberg and Hanahan, we know that despite the varieties of cancer, 6 driving forces lead to cancer cell survival. The "Hallmarks of cancer" result from:
1.Self sufficiency in growth signals: Cancer cells escape Anoikis, They secrete their own growth factors to achieve an autocrine stimulation.
2.Insensitivity to anti-growth signals. This is achieved by changing membranes' receptors composition and number, boosting its own global growth, and secreting Tumor Necrosis factors to tamper with surrounding cell machinery.
3.Sustained Angiogenesis, to maintain "feeding" of the new tumor mass. This is mostly critical for solid tumors. It is critical in tumors that bleed easily such as renal cell cancers.
4.Limitless replicative potential. By removing stops to mass formation, natural boundary sensors which contribute to shaping organs, Telomerase activation again.
5. Suppressing or escaping Apoptosis: By using cyclins and Bcl-2 and related molecules. Shielding Mitochondria and avoiding FAS/BAX, activating loopholes routes and impairing ubiquitination of growth molecules!
6.Tissue invasion and metastasis. Here the tumor cells alter composition, nature and amount of the cell receptors and adhsions molecules, cluster of differentiation (CD), and produce Tumor growth factors (TGF) which give it growth advantage vis-a-vis the surrounding tissue.
This list is by no mean exhaustive given the variety of possible oncogene mutations. However, when one gene is causing one of the 6 pathways, it is dubbed a DRIVER mutation for that cancer, and may have significant therapeutic importance.
This 6 venues are made of important molecular structures that can be a Target for therapy. Researcher are combing them one by one and targeting them. The successful experience with Multikinase therapy suggest that interrupting several points of the cascade appears beneficial. Computer models are being developed to see if sequential attacks or coordinated combinations would be better models for future therapies. The CRBCM is working to develop such a model. Our model will be complete after we enumerate all laws of nature (see our related series).
Model of cures should embrace these 6 venues in a mathematical equation...the challenge is launched!
Since the work of Weinberg and Hanahan, we know that despite the varieties of cancer, 6 driving forces lead to cancer cell survival. The "Hallmarks of cancer" result from:
1.Self sufficiency in growth signals: Cancer cells escape Anoikis, They secrete their own growth factors to achieve an autocrine stimulation.
2.Insensitivity to anti-growth signals. This is achieved by changing membranes' receptors composition and number, boosting its own global growth, and secreting Tumor Necrosis factors to tamper with surrounding cell machinery.
3.Sustained Angiogenesis, to maintain "feeding" of the new tumor mass. This is mostly critical for solid tumors. It is critical in tumors that bleed easily such as renal cell cancers.
4.Limitless replicative potential. By removing stops to mass formation, natural boundary sensors which contribute to shaping organs, Telomerase activation again.
5. Suppressing or escaping Apoptosis: By using cyclins and Bcl-2 and related molecules. Shielding Mitochondria and avoiding FAS/BAX, activating loopholes routes and impairing ubiquitination of growth molecules!
6.Tissue invasion and metastasis. Here the tumor cells alter composition, nature and amount of the cell receptors and adhsions molecules, cluster of differentiation (CD), and produce Tumor growth factors (TGF) which give it growth advantage vis-a-vis the surrounding tissue.
This list is by no mean exhaustive given the variety of possible oncogene mutations. However, when one gene is causing one of the 6 pathways, it is dubbed a DRIVER mutation for that cancer, and may have significant therapeutic importance.
This 6 venues are made of important molecular structures that can be a Target for therapy. Researcher are combing them one by one and targeting them. The successful experience with Multikinase therapy suggest that interrupting several points of the cascade appears beneficial. Computer models are being developed to see if sequential attacks or coordinated combinations would be better models for future therapies. The CRBCM is working to develop such a model. Our model will be complete after we enumerate all laws of nature (see our related series).
Model of cures should embrace these 6 venues in a mathematical equation...the challenge is launched!
Subscribe to:
Posts (Atom)