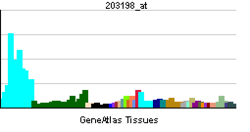
"Cytosolic complex---failure of estrogen receptor in triple negative breast cancer may lead to neoplastic transformation through this path!
The heparan sulfate on the surface of all adherent cells modulates the actions of a large number of extracellular ligands. Members of both cell surface heparan sulfate proteoglycan families, the transmembrane syndecans and the glycosylphosphoinositide-linked glypicans, bind these ligands and enhance formation of their receptor-signaling complexes. These heparan sulfate proteoglycans also immobilize and regulate the turnover of ligands that act at the cell surface. The extracellular domains of these proteoglycans can be shed from the cell surface, generating soluble heparan sulfate proteoglycans that can inhibit interactions at the cell surface.(bernfield et al)
In fact Heparan sulfate allows the progressive motility of Macrophages on cell/endothelial cell by giving and electrical/covalent liaisons (C-terminal) along the way
Heparin-binding EGF-like growth factor interacts with mouse blastocysts independently of ErbB1: a possible role for heparan sulfate proteoglycans and ErbB4 in blastocyst implantation
According to wikipediaWe have previously shown that the transcription factor Sp1 mediates the stimulatory effects of transforming growth factor-beta1 (TGF-beta1) on type IV collagen gene transcription and protein synthesis, and that estradiol reverses these effects by down-regulating Sp1 activity. Protein kinase casein kinase II (CK2) phosphorylates Egr-1 and prevents its binding to Sp1. We hypothesized that TGF-beta1 stimulates CK2 activity, which in turn activates type IV collagen gene transcription via increased availability of free Sp1.(Zdunek et al)
The aryl hydrocarbon receptor encodes a ligand-activated transcription factor involved in the regulation of biological responses to planar aromatic hydrocarbons. This receptor has been shown to regulate xenobiotic-metabolizing enzymes such as cytochrome P450. Its ligands included a variety of aromatic hydrocarbons. The Aryl hydrocarbon receptor (AhR or AHR) is a member of the family of basic helix-loop-helix transcription factors. The physiological ligands of this receptor are unknown (a proposed endogenous ligand is known, it is 2-(1′H-indole-3′-carbonyl)-thiazole-4-carboxylic acid methyl ester (ITE), which can operate in Dendritic cells, two articles:http://www.pnas.org/content/early/2010/11/09/1009201107, http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3396465/) , but it binds several exogenous ligands such as natural plant flavonoids, polyphenolics and indoles, as well as synthetic polycyclic aromatic hydrocarbons and dioxin-like compounds. AhR is a cytosolic transcription factor that is normally inactive, bound to several co-chaperones. Upon ligand binding to chemicals such as 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), the chaperones dissociate resulting in AhR translocating into the nucleus and dimerizing with ARNT (AhR nuclear translocator), leading to changes in gene transcription.
Non-ligand bound Ahr is retained in the cytoplasm as an inactive protein complex consisting of a dimer of Hsp90,[15][16] prostaglandin E synthase 3 (Ptges3, p23)[17][18][19][20] and a single molecule of the immunophilin-like protein hepatitis B virus X-associated protein 2 (XAP2),[21] which was previously identified as AhR interacting protein (AIP)[22] and AhR-activated 9 (ARA9).[23] The dimer of Hsp90, along with p23, has a multifunctional role in the protection of the receptor from proteolysis, constraining the receptor in a conformation receptive to ligand binding and preventing the premature binding of ARNT.[5][18][20][24][25][26] XAP2 interacts with carboxyl-terminal of Hsp90 and binds to the AhR nuclear localization sequence (NLS) preventing the inappropriate trafficking of the receptor into the nucleus.[27][28][29] wikipedia""promoter region of AhR responsive genes. The AhR/ARNT heterodimer directly binds the AHRE/DRE/XRE core sequence in an asymmetric manner such that ARNT binds to 5’-GTG-3’ and AhR binding 5’-TC/TGC-3’.[39][40] Recent research suggests that a second type of element termed AHRE-II, 5’-CATG(N6)C[T/A]TG-3’, is capable of indirectly acting with the AhR/ARNT complex.[41][42] Regardless of the response element, the end result is a variety of differential changes in gene expression'. The search for other metabolizing genes induced by Ahr ligands, due to the presence of DREs, has led to the identification of an "Ahr gene battery" of Phase I and Phase II metabolizing enzymes consisting of CYP1A1, CYP1A2, CYP1B1, NQO1, ALDH3A1, UGT1A2 and GSTA1.[58] Presumably, vertebrates have this function to be able to detect a wide range of chemicals, indicated by the wide range of substrates Ahr is able to bind and facilitate their biotransformation and elimination. The AhR may also signal the presence of toxic chemicals in food and cause aversion of such foods.[59]
AhR activation seems to be also important for immunological responses and inhibiting inflammation.[49][60] wikipedia
" In addition to the protein interactions mentioned above, AhR has also been shown to interact with RELA,[66][67] cyclin T1,[68]SRC-1,[69] retinoblastoma protein,[70] NRIP1,[71] estrogen receptor alpha,[72][73] NEDD8[74] and ARNTL.[75]
Direct interaction of c-Myc with Smad2 and Smad3 to inhibit TGF-beta-mediated induction of the CDK inhibitor p15(Ink4B).
Michael E. DeBakey Department of Surgery, Baylor College of Medicine, Houston, TX 77030, USA. xialin@bcm.tmc.edu
The c-Myc oncogene has been implicated in
the genesis of diverse human tumors. Ectopic expression of the c-Myc
gene in cultured epithelial cells causes resistance to the
antiproliferative effects of TGF-beta. However, little is known about
the precise mechanisms of c-Myc-mediated TGF-beta resistance. In this
study, we reveal that c-Myc physically interacts with Smad2 and Smad3,
two specific signal transducers involved in TGF-beta signaling. Through
its direct interaction with Smads, c-Myc binds to the Sp1-Smad complex
on the promoter of the p15(Ink4B) gene, thereby inhibiting the
TGF-beta-induced transcriptional activity of Sp1 and Smad/Sp1-dependent
transcription of the p15(Ink4B) gene. These results suggest that
oncogenic c-Myc promotes cell growth and cancer development partly by
inhibiting the growth inhibitory functions of Smads.
Mesh Terms:
Cell Cycle Proteins, Cell Line, Cyclin-Dependent Kinase Inhibitor p15, Cyclin-Dependent Kinase Inhibitor p16, Cyclin-Dependent Kinases, DNA-Binding Proteins, Humans, Promoter Regions, Genetic, Protein Binding, Proto-Oncogene Proteins c-myc, Receptors, Transforming Growth Factor beta, Signal Transduction, Smad2 Protein, Smad3 Protein, Sp1 Transcription Factor, Trans-Activators, Transforming Growth Factor beta, Tumor Suppressor Proteins
======================================================================Cell Cycle Proteins, Cell Line, Cyclin-Dependent Kinase Inhibitor p15, Cyclin-Dependent Kinase Inhibitor p16, Cyclin-Dependent Kinases, DNA-Binding Proteins, Humans, Promoter Regions, Genetic, Protein Binding, Proto-Oncogene Proteins c-myc, Receptors, Transforming Growth Factor beta, Signal Transduction, Smad2 Protein, Smad3 Protein, Sp1 Transcription Factor, Trans-Activators, Transforming Growth Factor beta, Tumor Suppressor Proteins
THESE ARE THE COMPELLING FACTS IN THE LITERATURE!
NEXT BLOG PUT ALL TOGETHER!