In all evidence, the receptor resistance can be induced by change in shape, change in molecular content, and and change in molecular nature of product with which it interacts. As it pertains to the Human Epidermal Growth factor Receptor or HER-2 Receptor, downstream interaction is with the PI3K/AKT/MTOR.
We now take it for granted as a truth that when,in a pathway, some thing clog the action, we target downstream steps. If you can't stop the enemy in the street, or gate, try stop him at your doorstep.
So instead of stopping the Her-2 Receptor, inhibit the MTOR, mammalian target of Rapamycin!
So now inhibiting the MTOR is a strategy to stop resistance or add to the effect of target therapy aimed at HER-2.
and guess what, it works! Proof of concept did materialize in clinical trials!
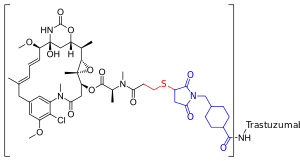
Scientist now are also taking the Her-2 Receptor targeting molecule, attach it to a powerful chemotherapy molecule and send the whole thing into the cell (concept of T-DM1).
Trastuzumab emtansine
From Wikipedia, the free encyclopedia
 |
|
|---|---|
| Monoclonal antibody | |
| Type | Whole antibody |
| Source | Humanized (from mouse) |
| Clinical data | |
| Trade names | Kadcyla |
| Pregnancy cat. | D (US) |
| Legal status | ℞-only (US) |
| Routes | Intravenous infusion |
| Pharmacokinetic data | |
| Bioavailability | N/A |
| Protein binding | 93% (in vitro) |
| Metabolism | Hepatic (CYP3A4/3A5-mediated) |
| Half-life | 4 days |
| Identifiers | |
| CAS number | 1018448-65-1 |
| ATC code | None |
| UNII | SE2KH7T06F |
| KEGG | D09980 |
| Chemical data | |
| Formula | C6448H9948N1720O2012S44·(C47H62ClN4O13S)n |
| Mol. mass | 148.5 kDa |
| |
|
In the EMILIA clinical trial of women with advanced HER2 positive breast cancer who were already resistant to trastuzumab alone, it improved survival by 5.8 months compared to the combination of lapatinib and capecitabine.[8] Based on that trial, the U.S. Food and Drug Administration (FDA) approved marketing on February 22, 2013.[9][10][11]
Trastuzumab emtansine was developed by Genentech. The planned cost is expected to be $9,800 a month, or $94,000 for a typical course of treatment.[10] wikipidea.
ANOTHER STRATEGY CREATED BY SCIENTIST!
Pertuzumab
From Wikipedia, the free encyclopedia
| Monoclonal antibody | |
|---|---|
| Type | Whole antibody |
| Source | Humanized (from mouse) |
| Target | HER2 |
| Clinical data | |
| Trade names | Perjeta; Omnitarg |
| Licence data | US FDA:link |
| Pregnancy cat. | D (US) |
| Legal status | ℞-only (US) |
| Routes | Intravenous |
| Identifiers | |
| CAS number | 380610-27-5 |
| ATC code | L01XC13 |
| UNII | K16AIQ8CTM |
| KEGG | D05446 |
| ChEMBL | CHEMBL2007641 |
| Chemical data | |
| Formula | ? |
| |
|

The structure of HER2 and pertuzumab
No comments:
Post a Comment